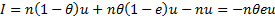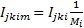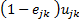For Bangladesh, the DALYs saved by AL as a first-line drug is 176,314*0.3%*91.7%/(1-0.3%*91.7%)=515.10.
References
1. Hassoun, N. The Global Health Impact Index: promoting global health. PLoS ONE 2015 Dec; 10(12): 10.1371/journal.pone.0141374. Available: journals.plos.org/plosone/article?id=10.1371/journal.pone.0141374.
2. Hassoun, N. Modeling key malaria drugs’ impact on global health: a reason to invest in the Global Health Impact Index. Am J Trop Med Hyg. Forthcoming.
3. Hassoun, N. Global health impact: A basis for labeling and licensing campaigns? Dev World Bioeth 2012 Dec; 12(3): 121-134.
4. Hassoun, N. Rating efforts to extend access on essential medicines: increasing global health impact. In: Lenard P, Straehle C, editors. Health Inequalities and Global Justice. Edinburg: Edinburgh University Press; 2012. pp. 176-196.
5. Hassoun, N. Globalization, global justice and global health impact. Public Aff Q 2014 Jul; 28(3): 231-258. Available: http://paq.press.illinois.edu/28/3/index.html. Accessed 1 Jan 2015.
6. About the Index. 2014 [cited 29 Dec 2015]. In: Global Health Impact Organization Website [internet]. Global Health Impact. Available: www.global-health-impact.org/aboutindex.php. Accessed 29 Dec 2015.
7. Eisele, T.P., Larsen, D.A., Walker, N., Cibulskis, R.E., Yukich, J.O., Zikusooka, C.M., Steketee, R.W. Estimates of child deaths prevented from malaria prevention scale-up in Africa 2001-2010. Malar J 2012; 11: 93. Available: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3350413/. Accessed 29 Dec 2015.
8. Galactionova, K., Tediosi, F., Savigny, D., Smith, T., Tanner, M. Effective coverage and systems effectiveness for malaria case management in Sub-Saharan African countries. PLoS One. 2015; 10(5): e0127818. Available: http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0127818. Access 15 Sep 2015.
9. Bhatt, S., Weiss, D.J., Cameron, E., Bisanzio, D., Mappin, B., Dalrymple, U., Battle, K.E., Moyes, C.L., Henry, A., Eckhoff, P.A., Wenger, E.A., Briët, O., Penny, M.A., Smith, T.A., Bennett, A., Yukich, J., Eisele, T.P., Griffin, J.T., Fergus, C.A., Lynch, M., Lindgren, F., Cohen, J.M., Murray, C.L.J., Smith, D.L., Hay, S.I., Cibulskis, R.E., Gething, P.W. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature 2015 Oct; 526(7572): 207-211.
10. Komatsu, R., Korenromp, E.L., Low-Beer, D., Watt, C., Dye, C., Steketee, R.W., Nahlen, B.L., Lyerla, R., Garcia-Calleja, J.M., Cutler, J., Schwartländer, B. Lives saved by global fund supported HIV/AIDS, tuberculosis and malaria programs: estimation approach and results between 2003 and end-2007. BMC Infect Dis Dec 2010; 10(109): 10.1186/1471-2334-10-109.
11. Bao, L. A new infectious disease model for estimating and projecting HIV/AIDS epidemics. Sex Transm Dis 2012 Dec; 88(Suppl_2): i58–i64. Available: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3512439/. Accessed 29 Dec 2015.
12. Malaria Treatment. Database: IndexMundi Data Portal [internet]. IndexMundi. Available: http://www.indexmundi.com/search.html?cx=partner-pub-5592729262323637%3Aj8a845havjo&cof=FORID%3A10&q=malaria+treatment&sa=Search&siteurl=www.indexmundi.com%2F&ref=www.indexmundi.com%2Fangola%2Fmalaria-treatment.html&ss=1783j236597j17. Accessed 29 Dec 2015.
13. World Health Organization. Global report on antimalarial drug efficacy and drug resistance: 2000-2010. Geneva: World Health Organization; 2010. Available: http://whqlibdoc.who.int/publications/2010/9789241500470_eng.pdf. Accessed 29 Dec 2015.
14. Priott, G., Kabakyenga, J., Pinoges, L., Ruiz, A., Eriksson, T., Coussement, F., Ngambe, T., Taylor, W.R., Perea, W., Guthmann, J.P., Olliaro, P., Legros, D. Artesunate and sulfadoxine-pyrimethamine combinations for the treatment of uncomplicated Plasmodium falciparum malaria in Uganda: a randomized, double-blind, placebo-controlled trial. Trans R Soc Trop Med Hyg 2003 May; 97(3): 325-330. Available: http://www.ncbi.nlm.nih.gov/pubmed/15228253?dopt=Abstract. Accessed 29 Dec 2015.
15. Yavo, W., Faye, B., Kuete, T., Djohan, V., Oga, S.A., Kassi R.R., Diatta, M., Ama, M.V., Tine, R., Ndiaye, J.L., Evi, J.B., Same-Ekobo, A., Faye, O., Koné, M. Multicentric assessment of the efficacy and tolerability of dihydroartemisinin-piperaquine compared to artemether-lumefantrine in the treatment of uncomplicated Plasmodium falciparum malaria in sub-Saharan Africa. Malar J 2011; 10: 198.
16. Tietche, F., Chelo, D., Mina Ntoto, N.K., Djoukoue, F.M., Hatz, C., Frey, S., Frentzel, A., Trapp, S., Zielonka, R., Mueller, EA. Tolerability and efficacy of a pediatric granule formulation of artesunate-mefloquine in young children from cameroon with uncomplicated falciparum malaria. Am J Trop Med Hyg 2010; 82(6): 1034-1040.
17. Tall, A., Rabarijaona L.P., Robert V., Bedja S.A., Ariey F. Randrianarivelojosia M. Efficacy of artesunate plus amodiaquine, artesunate plus sulfadoxine-pyrimethamine, and chloroquine plus sulfadoxine-pyrimethamine in patients with uncomplicated plasmodium falciparum in the Comoros Union. Acta Trop 2007; 102: 176-181.
18. The Four Artemisinin-Based Combinations (4ABC) Study Group. (A head-to-head comparison of four artemisinin-based combinations for treating uncomplicated malaria in African children: a randomized trial. PLoS Med 2011; 8: 1-16.
19. Valecha, N., Phyo, A. P., Mayxay, M., Newton, P. N., Krudsood, S., Keomany, S., Khanthavong, M., Pongvongsa, T., Ruangveerayuth, R., Uthaisil, C., Ubben, D., Duparc, S., Bacchieri, A., Corsi, M., Rao, B. H. K., Bhattacharya, P. C., Dubhashi, N., Ghosh, S. K., Dev, V. Kumar, Ashwani Pukrittayakamee, S. An open-label, randomised study of dihydroartemisinin-piperaquine versus artesunate-mefloquine for falciparum malaria in Asia. PLoS One 2010; 5: 1-13.
20. Anvikar, A., Anupkumar R., Sharma, B., Shahi, B. H., Tyagi, P. K., Bose, T. K., Sharma, S. K. Srivastava, P., Srivastava, B., Kiechel, J. R., Dash, A. P., Valecha, N. Artesunate-amodiaquine fixed dose combination for the treatment of plasmodium falciparum malaria in India. Malar J 2012; 11: 97-104.
21. Sagara, I., Diallo, A., Kone, M., Coulibaly, M., Diawara, S.I., Guindo, O, Maiga, H., Niambele M. B., Sissoko, M., Dicko, A., Djimde, A., Doumbo, O. K. A randomized trial of artesunate-mefloquine versus artemether-lumefantrine for treatment of uncomplicated plasmodium falciparum malaria in Mali. Am J Trop Med Hyg 2008; 79: 655-61.
22. Zwang, J., Ashley, E. A., Karema, C., D'Alessandro, U., Smithuis, F., Dorsey, G., Janssens, B., Mayxay, M., Newton, P., Singhasivanon, P., Stepniewska, K., White, N. J., Nosten, F. Safety and efficacy of dihydroartemisinin-piperaquine in falciparum malaria: a prospective multi-centre individual patient data analysis. PLoS One 2009; 4: 1-13.
23. World Health Organization. World malaria report 2011 annexes. Geneva: The World Health Organization; 2011. Available: http://www.who.int/malaria/world_malaria_report_2011/WMR2011_annexes_lowres.pdf. Accessed 29 Dec 2015.
24. World Health Organization. Data on the HIV/AIDS response: antiretroviral therapy coverage. Geneva: The World Health Organization; 2013. Available: http://apps.who.int/gho/data/node.main.574?lang=en. Accessed 29 Dec 2015.
25. Laurent, C., Kouanfack, C., Koulla-Shiro, S., Nkoué, N., Bourgeois, A., Calmy, A., Lactuock, B., Nzeusseu, V., Mougnutou, R., Peytavin, G., Liégeois, F., Nerrienet, E., Tardy, M., Peeters, M., Andrieux-Meyer, I., Zekeng, L., Kazatchkine, M., Mpoudi-Ngolé, E., Delaporte, E. Effectiveness and safety of a generic fixed-dose combination of nevirapine, stavudine, and lamivudine in HIV-1-infected adults in Cameroon: open-label multicentre trial. The Lancet 2004; 364: 29-34.
26. Manosuthi, W.,Tantanathip, P., Prasithisirikul, W., Likanonsakul, S., Sungkanuparph, S. Durability of stavudine, lamivudine and nevirapine among advanced HIV-1 infected patients with/without prior co-administration of rifampicin: a 144-week prospective study. BMC Infect Dis 2008; 8: 136-142.
27. Munderi, P., Walker, A.S., Kityo, C., Babiker, A.G., Ssali, F., Reid, A., Darbyshire, J.H., Grosskurth, H., Mugyenyi, P., Gibb, D.M., Gilks, C.F., DART/NORA trial teams. Nevirapine/zidovudine/lamivudine has superior immunological and virological responses not reflected in clinical outcomes in a 48-week randomized comparison with abacavir/zidovudine/lamivudine in HIV-infected Ugandan adults with low CD4 cell counts. HIV Med 2010; 11: 334–344.
28. Gallant, J., Staszewski, S., Pozniak, A.L., DeJesus, E., Suleiman, J.M., Miller, M.D., Coakley, D.F., Lu, B., Toole, J.J., Cheng, A.K., 903 Study Group. Efficacy and safety of tenofovir DF vs stavudine in combination therapy in antiretroviral-naive patients: a 3-year randomized trial. JAMA 2004; 292: 191-201.
29. Annan, N., Torshie, Nelson, M., Mandalia, S., Bower, M., Gazzard, B. G., Stebbing, J. The nucleoside backbone affects durability of efavirenz- or nevirapine-based highly active antiretroviral therapy in antiretroviral-naive individuals. J Acquir Immune Defic Syndr 2009; 51: 140-6.
30. Kebba, A., Atwine, D., Mwebaze, R., Kityo, C., Nakityo, R., Peter, M. Therapeutic responses to AZT + 3TC + EFV in advanced antiretroviral naive HIV type 1-infected Ugandan patients. AIDS Res Hum Retroviruses 2002; 18: 1181-7.
31. Cohen, C., Molina, J.M., Cahn, P., Clotet, B., Fourie, J., Grinsztejn, B., Wu, H., Johnson, M.A., Saag, M., Supparatpinyo, K., Crauwels, H., Lefebvre, E., Rimsky, L.T., Vanveggel, S., Williams, P., Boven, K., ECHO Study Group, THRIVE Study Group. Efficacy and safety of rilpivirine (TMC278) versus efavirenz at 48 weeks in treatment-naïve, HIV-1-infected patients: pooled results from the phase 3 double-blind, randomized ECHO and THRIVE trials. J Acquir Immune Defic Syndr 2012; 60: 33-42.
32. Sierra-Madero, J., Di Perri, G., Wood, R., Saag, M., Frank, I., Craig, C., Burnside, R., McCracken, J., Pontani, D., Goodrich, J., Heera, J., Mayer, H. Efficacy and safety of maraviroc versus efavirenz, both with zidovudine/lamivudine: 96-week results from the MERIT study. HIV Clin Trials 2010; 11: 125-32.
33. Gotuzzo, E., Markowitz, M., Ratanasuwan, W., Smith, G., Prada, G., Morales-Ramirez, J.O., Strohmaier, K.M., Lu, C., Bhanja, S., Nguyen, B.Y., Teppler, H., Protocol 004 Study Team. Sustained efficacy and safety of raltegravir after 5 years of combination antiretroviral therapy as initial treatment of HIV-1 infection: final results of a randomized, controlled, phase II study (Protocol 004). J Acquir Immune Defic Syndr 2012; 61: 73-7.
34. Markowitz, M., Nguyen, B.Y., Gotuzzo, E., Mendo, F., Ratanasuwan W., Kovacs, C., Prada, G., Morales-Ramirez, J.O., Crumpacker, C.S., Isaacs, R.D., Gilde, L.R., Wan, H., Miller, M.D., Wenning, L.A., Teppler, H., Protocol 004 Part II Study Team. Rapid and durable antiretroviral effect of the HIV-1 Integrase inhibitor raltegravir as part of combination therapy in treatment-naive patients with HIV-1 infection: results of a 48-week controlled study. J Acquir Immune Defic Syndr 2007; 46: 125–33.
35. DeJesus, E., Rockstroh, J.K., Lennox, J.L., Saag, M.S., Lazzarin, A., Zhao, J., Wan, H., Rodgers, A.J., Walker, M.L., Miller, M., DiNubile, M.J., Nguyen, B.Y., Teppler, H., Leavitt, R.,Sklar, P, STARTMRK Investigators. Efficacy of raltegravir versus efavirenz when combined with tenofovir/emtricitabine in treatment-naïve HIV-1-infected patients: week-192 overall and subgroup analyses from STARTMRK. HIV Clin Trials 2012; 13: 228-32.
36. Gallant, J., DeJesus, E., Arribas, J.R., Pozniak, A.L., Gazzard, B., Campo, R.E., Lu, B., McColl, D., Chuck, S., Enejosa, J., Toole, J.J., Cheng, A.K., Study 934 Group. Tenofovir DF, emtricitabine, and efavirenz vs. zidovudine, lamivudine, and efavirenz for HIV. N Engl J Med 2006; 354: 251–60.
37. Lennox, J., DeJesus, E., Lazzarin, A., Pollard, R.B., Madruga, J.V., Berger, D.S., Zhao, J., Xu, X., Williams-Diaz, A., Rodgers, A.J., Barnard, R.J., Miller, M.D., DiNubile, M.J., Nguyen, B.Y., Leavitt, R., Sklar, P., STARTMRK investigators. Safety and efficacy of raltegravir-based versus efavirenz-based combination therapy in treatment-naive patients with HIV-1 infection: a multicentre, doubleblind randomised controlled trial. The Lancet 2009; 374: 796–806.
38. Daar, E., Tierney, C., Fischl, M.A., Sax, P.E., Mollan, K., Budhathoki, C., Godfrey, C., Jahed, N.C., Myers, L., Katzenstein, D., Farajallah, A., Rooney, J.F., Pappa, K.A., Woodward, W.C., Patterson, K., Bolivar, H., Benson, C.A., Collier, A.C., AIDS Clinical Trials Group Study A5202 Team. Atazanavir plus ritonavir or efavirenz as part of a 3-drug regimen for initial treatment of HIV-1. Ann Intern Med 2011; 154: 445–56.
39. Post, F., Moyle, G.J., Stellbrink, H.J., Domingo, P., Podzamczer, D., Fisher, M., Norden, A.G., Cavassini, M., Rieger, A., Khuong-Josses, M.A., Branco, T., Pearce, H.C., Givens, N., Vavro, C., Lim, M.L. Randomized comparison of renal effects, efficacy, and safety with once-daily abacavir/lamivudine versus tenofovir/emtricitabine, administered with efavirenz, in antiretroviral-naive, HIV-1-infected adults: 48-week results from the ASSERT study. J Acquir Immune Defic Syndr 2010; 55: 49–57.
40. Puls, R., Puls, R.L., Srasuebkul, P., Petoumenos, K., Boesecke, C., Duncombe, C., Belloso, W.H., Molina, J.M., Li, L., Avihingsanon, A., Gazzard, B., Cooper, D.A., Emery, S., Altair Study Group. Efavirenz versus boosted atazanavir or zidovudine and abacavir in antiretroviral treatmentnaive, HIV-infected subjects: week 48 data from the Altair study. Clin Infect Dis 2010; 51: 855–64.
41. Campbell, T. B., Smeaton, L. M., Kumarasamy, N., Flanigan, T.,Klingman, K. L., Firnhaber, C., Grinsztejn, B., Hosseinipour, M. C., Kumwenda, J., Lalloo, U., Riviere, C., Sanchez, J., Melo, M., Supparatpinyo, K., Tripathy, S., Martinez, A. I., Nair, A., Walawander, A., Moran, L., Chen, Y., Snowden, W., Rooney, J. F., Uy, J., Schooley, R. T., De Gruttola, V., Hakim, J. G. Efficacy and safety of EFV of three antiretroviral regimens for initial treatment of HIV-1: randomized clinical trial in diverse multinational settings. PLoS Med 2012; 9: e1001290.
42. Miró, J., Manzardo, C., Ferrer, E., Loncà, M., Guardo, A.C., Podzamczer, D., Domingo, P., Curran, A., Clotet, B., Cruceta, A., Lozano, F., Pérez, I., Plana, M., Gatell, J.M., Advanz-3 Study Group. Immune reconstitution in severely immunosuppressed antiretroviral-naive HIV-1-infected patients taking antiretroviral regimens based on efavirenz, lopinavir-ritonavir, and atazanavir-ritonavir: 48-week results of a randomized controlled trial. AIDS Res Hum Retroviruses 2010; 26: 747-57.
43. Molina, J., Cahn, P., Grinsztejn, B., Lazzarin, A., Mills, A., Saag, M., Supparatpinyo, K., Walmsley, S., Crauwels, H., Rimsky, L. T., Vanveggel, S., Boven, K. Rilpivirine versus efavirenz with tenofovir and emtricitabine in treatment-naive adults infected with HIV-1 (ECHO): a phase 3 randomised double-blind active controlled trial. The Lancet 2011; 378: 238–46.
44. Landman, R., Poupard, M., Diallo, M., Ngom, G. N. F., Diakhate, N., Ndiaye, B., Toure, K. C., Trylesinski, A., Diop, H., Mboup, S., Koita, F., Delaporte, E., Benalycherif, A., Girard, P.M., Sow, P. S. Tenofovir-emtricitabineefavirenz in HIV-I-infected adults in Senegal: a 96-week pilot trial in treatment-naive patients. J Int Assoc Physicians AIDS Care 2009; 8: 379–84.
45. Rey, D., Hoen, B., Chavanet, P., Schmitt, M.P., Hoizey, G., Meyer, P., Peytavin, G., Spire, B., Allavena, C., Diemer, M., May, T., Schmit, J.L., Duong, M., Calvez, V., Lang, J.M. High rate of early virological failure with the once-daily tenofovir/lamivudine/nevirapine combination in naive HIV-1-infected patients. J Antimicrob Chemother 2009; 63: 380–8.
46. Soriano, V., Arastéh, K., Migrone, H., Lutz, T., Opravil, M., Andrade-Villanueva, J., Antunes, F., Di Perri, G., Podzamczer, D., Taylor, S., Domingo, P., Gellermann, H., de Rossi, L., ARTEN investigators. Nevirapine versus atazanavir/ritonavir, each combined with tenofovir disoproxil fumarate/emtricitabine, in antiretroviral-naive HIV-1 patients: the ARTEN Trial. Antivir Ther 2011; 16: 339–48.
47. Lockman, S., Hughes, J., McIntyre, Y., Zheng, T., Chipato, F., Conradie, F., Sawe, A., Asmelash, M.C., Hosseinipour, L., Mohapi, E., Stringer, R. Mngqibisa, A. Siika, D. Atwine, J. Hakim, D. Shaffer, C. Kanyama, K. Wools-Kaloustian, R.A. Salata, E., Hogg, B., Alston-Smith, A., Walawander, E., Purcelle-Smith, S., Eshleman, J., Rooney, S., Rahim, J.W., Mellors, R.T., Schooley, J.S. Currier for the OCTANE A5208 Study Team. Antiretroviral therapies in women after single-dose nevirapine exposure. N Engl J Med 2010; 363: 1499–509.
48. Vallecillo, G., Domingo, P., Mallolas, J., Blanch, J., Ferrer, E., Cervantes, M., Pedrol, E., Knobel, H., Llibre, J.M. Evaluation of the safety and effectiveness of nevirapine plus coformulated tenofovir/emtricitabine as first-line therapy in routine clinical practice. AIDS Res Hum Retroviruses 2012; 28: 165-70.
49. Bunupuradah, T., Chetchotisakd, P., Ananworanich, J., Munsakul, W., Jirajariyavej, S., Kantipong, P., Prasithsirikul, W., Sungkanuparph, S., Bowonwatanuwong, C., Klinbuayaem, V., Kerr, S.J., Sophonphan, J., Bhakeecheep, S., Hirschel, B., Ruxrungtham, K., HIV STAR Study Group. A randomized comparison of second-line lopinavir/ritonavir monotherapy vs. tenofovir/lamivudine/lopinavir/ritonavir in patients failing NNRTI-regimens: the HIV STAR study. Antivir Ther 2012; 17: 1351-61.
50. Puthanakit, T., van der Lugt, J., Bunupuradah, T., Ananworanich, J., Gorowara, M., Phasomsap, C., Jupimai, T., Boonrak, P., Pancharoen, C., Burger, D., Ruxrungtham, K. Pharmacokinetics and 48 week efficacy of low-dose lopinavir/ritonavir in HIV-infected children. J Antimicrob Chemother 2009; 64: 1080-6.
51. Smith, K., Patel, P., Fine, D., Bellos, N., Sloan, L., Lackey, P., Kumar, P.N., Sutherland-Phillips, D.H., Vavro, C., Yau, L., Wannamaker, P., Shaefer, M.S., HEAT Study Team. Randomized, double-blind, placebo-matched, multicenter trial of abacavir/lamivudine or tenofovir/emtricitabine with lopinavir/ritonavir for initial HIV treatment. AIDS 2009; 23: 1547-56.
52. Saez-Llorens, X., Violari, A., Ndiweni, D., Yogev, R., Cashat, M., Wiznia, A., Chittick, G., Harris, J., Hinkle, J., Blum, M.R., Adda, N., Rousseau, F., FTC-203 Study Team. Long-term safety and efficacy results of once-daily emtricitabine-based highly active antiretroviral therapy regimens in human immunodeficiency virus-infected pediatric subjects. Pediatrics 2008; 121: e827-35.
53. Murphy, R., da Silva, B.A., Hicks, C.B., Eron, J.J., Gulick, R.M., Thompson, M.A., McMillan, F., King, M.S., Hanna, G.J., Brun, S.C. Seven-year efficacy of a lopinavir/ritonavir-based regimen in antiretroviral-naïve HIV-1-infected patients. HIV Clin Trials 2008; 9: 1-10.
54. Echeverría, P., Negredo, E., Carosi, G., Gálvez, J., Gómez, J.L., Ocampo, A., Portilla, J., Prieto, A., López, J.C., Rubio, R., Mariño, A., Pedrol, E., Viladés, C., del Arco, A., Moreno, A., Bravo, I., López-Blazquez, R., Pérez-Alvarez, N., Clotet, B. Similar antiviral efficacy and tolerability between efavirenz and lopinavir/ritonavir, administered with abacavir/lamivudine (Kivexa), in antiretroviral-naïve patients: a 48-week, multicentre, randomized study (Lake Study). Antiviral Res 2010; 85: 403-8.
55. Pensi, T. Fixed dose combination of lamivudine, stavudine and nevirapine in the treatment of pediatric HIV infection: a preliminary report. Indian Pediatr 2007; 44: 519-21.
56. Frange P., Briand, N., Avettand-fenoel, V., Veber, F., Moshous, D., Mahlaoui, N., Rouzioux, C., Blanche, S., Chaix, M.L. Lopinavir/ritonavir-based antiretroviral therapy in human immunodeficiency virus type 1-infected naive children: rare protease inhibitor resistance mutations but high lamivudine/emtricitabine resistance at the time of virologic failure. Pediatr Infect Dis J 2011; 30: 684-8.
57. World Health Organization. Antiretroviral medicines in low- and middle-income countries: usage in 2010 with global and regional demand forecast for 2011–2012. Geneva: The World Health Organization; 2013. Available: http://www.who.int/hiv/pub/amds/2013forecast_report/en/. Accessed 29 Dec 2015.
58. World Health Organization. Data for global tuberculosis control. Geneva: The World Health Organization. Available: http://who.int/tb/country/data/download/en/index.html. Accessed 29 Dec 2015.
59. World Health Organization. Global tuberculosis control 2011. Geneva: The World Health Organization; 2011. Available: http://apps.who.int/iris/bitstream/10665/44728/1/9789241564380_eng.pdf. Accessed 29 Dec 2015.
60. World Health Organization. Multidrug-resistant tuberculosis 2013 Update. Geneva: The World Health Organization; 2013. Available: http://www.who.int/tb/challenges/mdr/MDR_TB_FactSheet.pdf. Accessed 29 Dec 2015.
61. World Health Organization. 2007-2008 XDR & MDR Tuberculosis global response plan. Geneva: The World Health Organization; 2007. Available: http://www.who.int/tb/challenges/xdr/xdr_mdr_factsheet_2007_en.pdf. Accessed 29 Dec 2015.
62. World Health Organization. (2013). Global tuberculosis report 2013. Geneva: The World Health Organization; 2013. Available: http://apps.who.int/iris/bitstream/10665/91355/1/9789241564656_eng.pdf?ua=1. Accessed 29 Dec 2015.
63. Institute for Health Metrics and Evaluation. The global burden of disease: generating evidence: guiding policy. Seattle: University of Washington; 2013. Available: http://www.healthdata.org/sites/default/files/files/policy_report/2013/GBD_GeneratingEvidence/IHME_GBD_GeneratingEvidence_FullReport.pdf. Accessed 29 Dec 2015.
64. World Health Organization. Global price reporting mechanism for HIV, tuberculosis and malaria. In: World Health Organization Website [internet]. Geneva: World Health Organization; 2016. Available: http://www.who.int/hiv/amds/gprm/en/. Accessed 1 Jan 2016.
65. Silverman, E. A. New index measures impact pharma has on infectious diseases. The Wall Street Journal Pharmalot. 23 Jan 2015. Available: http://blogs.wsj.com/pharmalot/2015/01/23/a-new-index-measures-impact-pharma-has-on-infectious-diseases/. Accessed 29 Dec 2015.
66. Prakash N. SUNY Professor Indexes Pharma Companies' Impact. POLITICO New York beta. 23 Jan 2015. Available: http://www.capitalnewyork.com/article/albany/2015/01/8560712/suny-professor-indexes-pharma-companies-impact. Accessed 29 Dec 2015.
67. Gorenstein D. New effort ranks drugmakers by impact. American Public Media Marketplace: Health Care. 23 Jan 2015. Available: http://www.marketplace.org/topics/health-care/new-effort-ranks-drug-makers-impact. Accessed 29 Dec 2015.
68. Berman J. Web Tool Tracks Locations of Vital Drugs' Biggest Impact. Voice of America Health. 15 Dec 2015. Available: http://www.voanews.com/content/new-web-tool-identifies-drug-companies-helping-poor/3104287.html. Accessed 29 Dec 2015.
69. UN Secretary-General’s Independent Expert Advisory Group on a Data Revolution for Sustainable Development. A world that counts: mobilising the data revolution for sustainable development. November 2014. Available: http://www.undatarevolution.org/wp-content/uploads/2014/12/A-World-That-Counts2.pdf. Accessed 29 Dec 2015.
70. World Bank Group and International Monetary Fund. Global monitoring report 2015/2016: development goals in an era of demographic change. Advance Edition. Washington, DC: International Bank for Reconstruction and Development / The World Bank; 2016. p. 107, Box 2.3. Available: http://pubdocs.worldbank.org/pubdocs/publicdoc/2015/10/503001444058224597/Global-Monitoring-Report-2015.pdf. Accessed 29 Dec 2015.
71. Trouiller, P., Torreele, E., Olliaro, P., White, N., Foster, S., Wirth, D., Pécoul, B. Drugs for neglected diseases: a failure of the market and a public health failure? Trop Med Int Health 2002; 6: 945-51.
72. Chatterjee, S., Zheng Y. A survey on consumers’ willingness to purchase things with a Global Health Impact label. Paper under preparation. Binghamton University, 2014.
73. World Health Organization. Public health-innovation and intellectual property rights: report of the commission on intellectual property rights, innovation and public health. Geneva: The World Health Organization; 2006. Available: http://www.who.int/intellectualproperty/documents/thereport/ENPublicHealthReport.pdf. Accessed 29 Dec 2015.
74. Futures Group. 2015 [cited 29 Dec 2015]. Spectrum. In: Futures Group Website, Resources, Software/Models [internet]. Futures Group Global. Available: http://futuresgroup.com/resources/software_models/spectrum. Accessed 29 Dec 2015.
75. Stover, J. AIM: a computer program for making HIV/AIDS projections and examining the demographic and social impacts of AIDS. Washington, DC: Futures Group International, United States Agency of International Development Health Policy Initiative; 2009. pp. 4-5. Available: http://www.healthpolicyinitiative.com/Publications/Documents/1253_1_AimManE.pdf. Accessed 29 Dec 2015.
76. Access to Medicine Index. The access to medicine index 2012. Haarlem: Access to Medicine Foundation, 2012. Available: http://www.accesstomedicineindex.org/sites/2015.atmindex.org/files/2012-access-to-medicine-index-clickable.pdf. Accessed 29 Dec 2015.
77. Murray, C. J. L., Vos, T., Lozano, R., Naghavi, M., Flaxman, A. D., Michaud, C., Ezzati, M., Shibuya, K., Salomon, J. A., Abdalla, S., Aboyans, V., Abraham, J., Ackerman, I., Aggarwal, R., Ahn, S. Y., Ali, M. K., AlMazroa, M. A., Alvarado, M., Anderson, H. R., Anderson, L. M., Andrews, K. G., Atkinson, C., Baddour, L. M., Bahalim, A. N., Barker-Collo, S., Barrero, L. H., Bartels, D. H., Basáñez, M., Baxter, A., Bell, M. L., Benjamin, E. J., Bennett, D., Bernabé, E., Bhalla, K., Bhandari, B., Bikbov, B., Abdulhak, A., Birbeck, G., Black, J. A., Blencowe, H., Blore, J. D., Blyth, F., Bolliger, I., Bonaventure, A., Boufous, S., Bourne, R., Boussinesq, M., Braithwaite, T., Brayne, C., Bridgett, L., Brooker, S., Brooks, P., Brugha, T. S., Bryan-Hancock, C., Bucello, C., Buchbinder, R., Buckle, G., Budke, C. M., Burch, M., Burney, P., Burstein, R., Calabria, B., Campbell, B., Canter, C. E., Carabin, H., Carapetis, J., Carmona, L., Cella, C., Charlson, F., Chen, H., Cheng, A. T., Chou, D., Chugh, S. S., Coffeng, L. E., Colan, S. D., Colquhoun, S., Colson, K. E., Condon, J., Connor, M. D., Cooper, L. T., Corriere, M., Cortinovis, M., de Vaccaro, K. C., Couser, W., Cowie, B. C., Criqui, M. H., Cross, M., Dabhadkar, K. C., Dahiya, M., Dahodwala, N., Damsere-Derry, J., Danaei, G., Davis, A., Leo, D. D., Degenhardt, L., Dellavalle, R., Delossantos, A., Denenberg, J., Derrett, S., Des Jarlais, D. C., Dharmaratne, S. D., Dherani, M., Diaz-Torne, C., Dolk, H., Dorsey, E. R., Driscoll, T., Duber, H., Ebel, B., Edmond, K., Elbaz, A., Ali, S. E., Erskine, H., Erwin, P. J., Espindola, P., Ewoigbokhan, S. E., Farzadfar, F., Feigin, V., Felson, D. T., Ferrari, A., Ferri, C. P., Fèvre, E. M., ; Finucane, M. M., Flaxman, S., Flood, L., Foreman, K., Forouzanfar, M. H., Fowkes, F. G. R., Fransen, M., Freeman, M. K., Gabbe, B. J., Gabriel, S. E., Gakidou, E., Ganatra, H. A., Garcia, B., Gaspari, F., Gillum, R. F., Gmel, G., Gonzalez-Medina, D., Gosselin, R., Grainger, R., Grant, B., Groeger, J., Guillemin, F., Gunnell, D., Gupta, R., Haagsma, J., Hagan, H., Halasa, Y. A., Hall, W., Haring, D., Haro, J. M., Harrison, J. E., Havmoeller, R., Hay, Roderick J. H. H., Hill, C., Hoen, B., Hoffman, H., Hotez, P. J., Hoy, D., Huang, J. J., Ibeanusi, S. E., Jacobsen, K. H., James, Spencer L., Jarvis, D., Jasrasaria, R., Jayaraman, S., Johns, N., Jonas, Jost B., Karthikeyan, G., Kassebaum, N., Kawakami, N., Keren, A., Khoo, J., King, C. H., Knowlton, L. M., Kobusingye, O., Koranteng, A., Krishnamurthi, R., Laden, F., Lalloo, R., Laslett, L. L., Lathlean, T., Leasher, J. L., Lee, Y. Y., Leigh, J., Levinson, D., Lim, S. S., Limb, E., Lin, J. K., Lipnick, M., Lipshultz, S. E., Liu, W., Loane, M., Ohno, S. L., Lyons, R., Mabweijano, J., MacIntyre, M. F., Malekzadeh, R., Mallinger, L., Manivannan, S., Marcenes, W., March, L., Margolis, D. J., Marks, G. B., Marks, R., Matsumori, A., Matzopoulos, R., Mayosi, B. M., McAnulty, J. H., McDermott, M. M., McGill, N., McGrath, J., Medina-Mora, M. E., Meltzer, M., Memish, Z. A., Mensah, G. A., Merriman, T. R., Meyer, A., Miglioli, V., Miller, M., Miller, T. R., Mitchell, P. B., Mock, C., Mocumbi, A. O., Moffitt, T. E., Mokdad, A. A., Monasta, L., Montico, M., Moradi-Lakeh, M., Moran, A., Morawska, L., Mori, R., Murdoch, M. E., Mwaniki, M. K., Naidoo, K., Nair, M. N., Naldi, L., Narayan, K.M.V., Nelson, P. K., Nelson, R. G., Nevitt, M. C., Newton, C. R., Nolte, S., Norman, P., Norman, R., O'Donnell, M., O'Hanlon, S., Olives, C., Omer, S. B., Ortblad, K., Osborne, R., Ozgediz, D., Page, A., Pahari, B., Pandian, J. D., Rivero, A. P., Patten, S. B., Pearce, N., Padilla, R. P., Perez-Ruiz, F., Perico, N., Pesudovs, K., Phillips, D., Phillips, M. R., Pierce, K., Pion, S., Polanczyk, G. V., Polinder, S., Pope, C. A., Popova, S., Porrini, E., Pourmalek, F., Prince, M., Pullan, R. L., Ramaiah, K. D., Ranganathan, D., Razavi, H., Regan, M., Rehm, J. T., Rein, D. B., Remuzzi, G., Richardson, K., Rivara, F. P., Roberts, T., Robinson, C., De Leòn, F. R., Ronfani, L., Room, R., Rosenfeld, L. C., Rushton, L., Sacco, R. L., Saha, S., Sampson, U., Sanchez-Riera, L., Sanman, E., Schwebel, D. C., Scott, J. G., Segui-Gomez, M., Shahraz, S., Shepard, D. S., Shin, H., Shivakoti, R., Silberberg, D., Singh, D., Singh, G., Singh, J.A., Singleton, J., Sleet, D. A., Sliwa, K., Smith, E., Smith, J. L., Stapelberg, N.J.C., Steer, A., Steiner, T., Stolk, W. A., Stovner, L. J., Sudfeld, C., Syed, S., Tamburlini, G., Tavakkoli, M., Taylor, H. R., Taylor, J. A., Taylor, W. J.,Thomas, B., Thomson, W. M.,Thurston, G. D., Tleyjeh, I. M., Tonelli, M., Towbin, J. A., Truelsen, T., Tsilimbaris, M. K., Ubeda, C., Undurraga, E. A., van der Werf, M. J., van Os, J., Vavilala, M. S., Venketasubramanian, N., Wang, M., Wang, W., Watt, K., Weatherall, D. J., Weinstock, M. A.,Weintraub, R., Weisskopf, M. G., Weissman, M. M., White, R. A., Whiteford, H., Wiebe, N., Wiersma, S.T., Wilkinson, J. D., Williams, H.C., Williams, S.R.M., Witt, E.,Wolfe, F.,Woolf, A. D., Wulf, S., Yeh, P. H., Zaidi, A. K. M., Zheng, Z., Zonies, D., Lopez, A.D. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. The Lancet 2013; 380: 2197-2223.
78. Global Health Data Exchange (GHDx). Country Profiles. Global Burden of Disease Study 2010 (GBD 2010) Results 1990-2010. Seattle: Institute for Health Metrics and Evaluation. Available: http://ghdx.healthmetricsandevaluation.org/country_profiles. Accessed 29 Dec 2015.
79. World Health Organization. World malaria report 2013 country profiles. Geneva: World Health Organization; 2013. Available: http://www.who.int/malaria/publications/world_malaria_report_2013/wmr2013_country_profiles.pdf?ua=1. Accessed 29 Dec 2015.
80. About UNICEF data and analytics. 2015. In: UNICEF Data Website [internet]. UNICEF. Available: http://www.data.unicef.org/indexd0c9.html?section=unicef_aboutus. Accessed 29 Dec 2015.
81. World Health Organization. Global report on antimalarial drug efficacy and drug resistance: 2000-2010. Geneva: World Health Organization; 2010. p. 98. Available: http://whqlibdoc.who.int/publications/2010/9789241500470_eng.pdf. Accessed 29 Dec 2015.
82. World Health Organization, UNICEF, UNAIDS. Global Update on HIV Treatment 2013: Results, Impact, and Opportunities. Geneva: The World Health Organization; 2013.
83. Meeting report: joint WHO/UNAIDS Annual Consultation with Pharmaceutical Companies and Stakeholders on Forecasting Global Demand of Antiretroviral Drugs for 2013-2016. 25-26 November, 2013, Geneva, Switzerland. Geneva: The World Health Organization; 2014.
84. Pan American Health Organization, World Health Organization Regional Office for the Americas. Antiretroviral Treatment in the Spotlight: A Public Health Analysis in Latin America and the Caribbean. Washington, D.C.: Regional Office for the Americas of the World Health Organization; 2014.
85. Disease Tutorials: HIV Tutorial 2-f. Survival Time on ART. 2015 [cited 29 Dec 2015]. In: Institute for Disease Modeling Website [internet]. Intellectual Ventures Property Holdings, LLC. Available: http://idmod.org/idmdoc/Content/EMOD/DiseaseTutorials/STI_and_HIV_Tutorials/2f_Survival_Time_on_ART.htm. Accessed 29 Dec 2015.
86. Delva, W. Coverage strategies: insights from population simulation models. International center for reproductive health and South African center for epidemiological modelling and analysis. In: Center for Effective Global Action, University of California, Berkeley Website [internet]. Available: http://cega.berkeley.edu/assets/cega_events/33/1.11_Coverage_Strategies_Delva.pdf. Accessed 29 Dec 2015.
87. Gadpayle, A.K., Kumar, N., Duggal, A., Rewari, B.B., Ravi, V. Survival trend and prognostic outcome of AIDS patients according to age, sex, stages, and mode of transmission – A retrospective study at ART centre of a tertiary care hospital. JIACM 2012; 13(4): 291-8. Available: http://medind.nic.in/jac/t12/i4/jact12i4p291.pdf. Accessed 29 Dec 2015.
88. World Health Organization. Technical report: Access to Antiretroviral Drugs in low- and middle- income countries. Geneva: World Health Organization; 2014. Available: http://apps.who.int/iris/bitstream/10665/128150/1/9789241507547_eng.pdf?ua=1&ua=1. Accessed 1 Jan 2016.
89. Waning, B., Diedrichsen, E., Moon, S. A lifeline to treatment: the role of Indian generic manufacturers in supplying antiretroviral medicines to developing countries. J Int AIDS Soc 2010 Sep 14; 13: 35. Available: http://www.ncbi.nlm.nih.gov/pubmed/20840741. Accessed 15 Sep 2015.
90. World Health Organization. World malaria report 2012. Geneva: The World Health Organization; 2012. Available: http://www.who.int/malaria/publications/world_malaria_report_2012/en/. Accessed 29 Dec 2015.
91. Kedir, A.A., Desta, A., Fesseha, G. Factors affecting survival of HIV positive children taking antiretroviral therapy at Adama Referral Hospital and Medical College, Ethiopia. J AIDS Clin Res 2014; 5(3): 289. Available: http://www.omicsonline.org/open-access/factors-affecting-survival-of-hiv-positive-children-taking-antiretroviral-therapy-at-adama-referral-hospital-and-medical-college-ethiopia-2155-6113.1000289.pdf. Accessed 29 Dec 2015.
92. Ayalew, J., Moges, H., sahu, O., Worku, A. Identifying factors related to the survival of aids patients under the follow-up of antiretroviral therapy (ART): the case of South Wollo. International Journal of Data Envelopment Analysis and *Operations Research* 2014 1(2): 21-27. Available: http://pubs.sciepub.com/ijdeaor/1/2/2/. Accessed 29 Dec 2015.
93. Cornell, M., Schomaker, M., Garone, D.B., Giddy, J., Hoffmann, C.J., Lessells, R., Maskew M., Prozesky H., Wood R., Johnson L.F., Egger M., Boulle A., Myer L., International Epidemiologic Databases to Evaluate AIDS Southern Africa (IeDEA-SA) Collaboration. Gender differences in survival among adult patients starting antiretroviral therapy in south africa: a multicentre cohort study. PLoS Medicine 2012; 9(9): e1001304.
94. Taylor-Smith, K., Tweya, H., Harries, A., Schoutene, E., Jahn, A. Gender differences in retention and survival on antiretroviral therapy of HIV-1 infected adults in Malawi. Malawi Med J 2010; 22(2): 49–56. Available: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3345762/. Accessed 29 Dec 2015.
95. Mitnick, C., Sonya, S., Kwonjune, J. S., Rich, M. L., Atwood, S. S., Furin, J. J., Fitzmaurice, G. M., Alcantara Viru, F. A., Appleton, S. C., Bayona, J. N., Bonilla, C. A., Chalco, K., Choi, S., Franke, M. F., Fraser, H. S. F., Guerra, D., Hurtado, R. M., Darius, J., Joseph, K., Llaro, K., Mestanza, L., Mukherjee, J. S., Muñoz, M., Palacios, E., Sanchez, E., Sloutsky, A., Becerra, M. C. Comprehensive treatment of extensively drug-resistant tuberculosis. N Engl J Med 2008; 359: 563-574.
96. Mphahlele, M., Syre, H., Valvatne, H., Stavrum, R., Mannsåker, T., Muthivhi, T., Weyer, K., Fourie, P.B., Grewal, H.M. Pyrazinamide resistance among South African multidrug-resistant mycobacterium tuberculosis isolates. J Clin Microbiol 2008; 46: 3459-3464.
97. Lafferty, J., Wasserman, L. Statistical analysis of semi-supervised regression. Carnegie Mellon University Research Showcase; 2007. Available: http://repository.cmu.edu/cgi/viewcontent.cgi?article=2031&context=compsci. Accessed 29 Dec 2015.
98. Committee on Economic, Social and Cultural Rights (CESCR). 1990. General Comment 3: The Nature of States Parties’ Obligations, Fifth Session, UN Doc. E/1991/23, annex III at 86(1991) Reprinted in Compilation of General Comments and General Recommendations Adapted by Human Rights Treaty Bodies, UN Doc. HRI/Gen/I/Rev. 6 at 62 (2003). http://www1.umn.edu/humanrts/gencomm/epcomm3.htm.
99. Gostin, Lawrence, et al. 2012. Towards a Framework Convention on Global Health. Bulletin of the World Health Organization.http://www.who.int/bulletin/volumes/91/10/12-114447/en/.
100. Gostin, Lawrence. 2001. The Human Right to Health: A Right to the ‘Highest Attainable Standard of Health. The Hastings Center Report 31 (2): 29-30.
101. United Nations (UN). 1966. Universal Declaration of Human Rights (UNDHR). International Covenant on Economic, Social and Cultural Rights Office of the High Commissioner of Human Rights. http://www.ohchr.org/EN/ProfessionalInterest/Pages/CESCR.aspx.
102. Ruggie, John. 2008. Protect, Respect and Remedy: A Framework for Business and Human Rights Report of the Special Representative of the Secretary-General on the Issue of Human Rights and Transnational Corporations and Other Business Enterprises, Human Rights Council 8 (3). P. 18.
103. Hunt, Paul. 2008. Human Rights Guidelines for Pharmaceutical Companies in Relation to Access to Medicines. United Nations (August 11)
104. Lee, Joo Young and Paul Hunt. 2012. Human Rights Responsibilities of Pharmaceutical Companies in Relation to Access to Medicines. Journal of Law Medicine & Ethics 40 (2): 225.
105. Zhou, Z.H., Li, M. 2005. Semi-supervised regression with co-training. In: International Joint Conferences on Artificial Intelligence 2005 Proceedings Website [internet]. pp. 908-913. Available: http://www.ijcai.org/search.php. Accessed 29 Dec 2015.
106. World Bank Group and International Monetary Fund. Global monitoring report 2015/2016: development goals in an era of demographic change. Advance Edition. Washington, DC: International Bank for Reconstruction and Development / The World Bank; 2016. Available: http://pubdocs.worldbank.org/pubdocs/publicdoc/2015/10/503001444058224597/Global-Monitoring-Report-2015.pdf. Accessed 29 Dec 2015.
107. Goal: Achieve universal primary education. UNICEF Millenium Development Goals Website [internet]. Available: http://www.unicef.org/mdg/index_education.htm. Accessed 29 Dec 2015.
108. Goal: Promote gender equality and empower women. UNICEF Millenium Development Goals Website [internet]. Available: http://www.unicef.org/mdg/index_genderequality.htm. Accessed 29 Dec 2015.
109. Cutler, D., Lleras-Muney, A. Policy Brief #9: Education and Health. Mar 2007 [cited 29 Dec 2015]. In: The University of Michigan National Poverty Center Website. Ann Arbor: The University of Michigan. Available: http://www.npc.umich.edu/publications/policy_briefs/brief9/. Accessed 29 Dec 2015.
110. Health and Academics. 1 Sep 2015 [cited 29 Dec 2015]. Centers for Disease Control and Prevention Website [internet]. Atlanta: U.S. Department of Health & Human Services. Available: http://www.cdc.gov/HealthyYouth/health_and_academics/. Accessed 29 Dec 2015.
111. World Bank Group and International Monetary Fund. Global monitoring report 2015/2016: development goals in an era of demographic change. Advance Edition. Washington, DC: International Bank for Reconstruction and Development / The World Bank; 2016. pp. 89-93. Available: http://pubdocs.worldbank.org/pubdocs/publicdoc/2015/10/503001444058224597/Global-Monitoring-Report-2015.pdf. Accessed 29 Dec 2015.
112. Lochner, L. Non-productive benefits of education: crime, health, and good citizenship. NBER Working Paper 16722. Cambridge, MA: National Bureau of Economic Research, Inc.; 2011. Available: http://www.nber.org/papers/w16722.pdf. Accessed 29 Dec 2015.
113. Alderman, H., Bleakley, H. Child health and educational outcomes. In Glewwe, P., editor. Education policy in developing countries. Chicago: The University of Chicago Press; 2014. pp. 107-136.
114. Strickland, B. Designing effective education programs for school health in developing countries compendium. United States Agency of International Development Educational Quality Improvement Program; 2011. Available: http://www.equip123.net/docs/E1-FP_Health_Comp_Web.pdf. Accessed 29 Dec 2015.
115. How We Work. 2015 [cited 29 Dec 2015]. In: Save the Children Organization Website [internet]. Fairfield: Save the Children. Available: http://www.savethechildren.org/site/c.8rKLIXMGIpI4E/b.6196517/k.9825/How_We_Work.htm. Accessed 29 Dec 2015.
116. Johnson & Johnson. 28 Dec 2015 [cited 29 Dec 2015]. In: Johnson & Johnson Company Website. Johnson & Johnson Services, Inc. Available: http://www.jnj.com/. Accessed 29 Dec 2015.
117. PHARMACEUTICALS: Facts, Policies and NCSL Resources. Nov 2015 [cited 29 Dec 2015]. In: National Conference of State Legislatures Website [internet]. Denver: National Conference of State Legislatures Website. Available: http://www.ncsl.org/research/health/pharmaceuticals-facts-policies-and-ncsl-resources.aspx. Accessed 29 Dec 2015.
118. Country Reports. 2015 [cited 29 Dec 2015]. In PMLiVE Website [internet]. PMGroup Worldwide Ltd. Available: http://www.pmlive.com/intelligence/country_reports. Accessed 29 Dec 2015.
119. Top 25 Pharma Companies by Global Sales. 2015 [cited 29 Dec 2015]. In PMLiVE Website [internet]. PMGroup Worldwide Ltd. Available: http://www.pmlive.com/top_pharma_list/global_revenues. Accessed 29 Dec 2015.
120. Drugs. 29 Dec 2015 [cited 29 Dec 2015]. In: U.S. Food and Drug Administration Website [internet]. Silver Spring: U.S. Food and Drug Administration. Available: http://www.fda.gov/Drugs/default.htm. Accessed 29 Dec 2015.
121. The University of Chicago Medicine. 2015 [cited 29 Dec 2015]. University of Chicago Hospitals Website [internet]. Chicago: The University of Chicago Medical Center. Available: http://www.uchospitals.edu/. Accessed 29 Dec 2015.
122. Walgreens. 2015 [cited 29 Dec 2015]. In: Walgreens Company Website [internet]. Deerfield: Walgreen Co. Available: http://www.walgreens.com/. Accessed 29 Dec 2015.
123. Learn About Glu. 2015 [cited 29 Dec 2015]. In: T1D Exchange for Type 1 Diabetes Website [internet]. Available: https://myglu.org/. Accessed 29 Dec 2015.
124. Zhongfei, Z., Ruofei, Z. Multimedia Data Mining – A Systematic Introduction to Concepts and Theory. Boca Raton: Taylor & Francis Group/CRC Press; 2008.
125. Bo L., Zhongfei, Z., Philip, S. Y. Relational data clustering: models, algorithms, and applications. Boca Raton: Taylor & Francis/CRC Press; 2010.
126. Zhen G., Zhongfei Z., Shenghuo, Z., Yun, C., Yihong, G. A two-level topic model towards knowledge discovery from citation networks, ieee transactions on knowledge and data engineering. IEEE Computer Society Press 2014; 26(4): 780 – 794.
127. GHI Spreadsheet ORS v63. In: Open Science Framework Website [internet]. Center for Open Science; 2015. Available: https://osf.io/zghvy/. Accessed 1 Jan 2016.
128. Awards and Recognition. 11 Dec 2015 [cited 29 Dec 2015]. In: Sanofi Company Website [internet]. Sanofi. Available: http://www.sanofi.us/l/us/en/layout.jsp?scat=B4A58C45-AA7A-4526-84A4-6E26FED0C640. Accessed 29 Dec 2015.
129. World Health Organization and World Bank. Monitoring Progress towards Universal Health Coverage at Country and Global Levels: A Framework. 2013; 1-10.
130. United Nations (UN). Sustainable Development Goals. 2015. http://www.un.org/sustainabledevelopment/sustainable-development-goals/.
131. Walker, N., Tam, Y., and Ingrid K Friberg. Overview of the Lives Saved Tool (LiST). BMC Public Health 2013;13 (Suppl 3): S1.
132. Gostin L. Global health law. Cambridge: Harvard University Press; 2014.
133. High-level panel of eminent persons on the post-2015 development. Bali Communiqué of the High-Level Panel, March 28, 2013.