Abstract
TRADE RELATED INTELLECTUAL PROPERTY RIGHTS AND ACCESS TO ESSENTIAL MEDICINES FOR NON-COMMUNICABLE DISEASES WITH A FOCUS ON SOUTH AFRICA
Seema Rath1 and Sunitha Srinivas2
ABSTRACT
The access to essential medicines is accorded vital importance in addressing the problem of communicable diseases and the epidemic rise in non-communicable diseases. Access to affordable essential medicines for chronic diseases is necessaryto eradicate poverty and inequity. However, several policy and implementation incoherencies such as, TRIPS-plus agenda of evergreening and data exclusivity have a negative impact on accessibility of affordable medicines, which are a major hindrance to advancing human rights and achieving sustainable development. South Africa, despite being an upper middle income country, faces high levels of poverty and inequality, and low life expectancy. Even though, the Constitutional recognition of Health as a Human Right, and access to affordable essential medicines for all, there is lack of availability of essential medicines for non-communciable diseases in its public facilities. South Africa has a depository patent registration system rather than an examination system, leading to liberal grant of patents. This restricts the entry of innovators and generic manufacturers leading monopoly control. Its medicine prices in the private facilities are one of the most expensive ones in the world. Despite adequate policies in South Africa, like many other LMICs, the gap between policy and practices are expanding. Hence, there is a need to modify national patent laws to facilitate access to essential medicines. By proactively incorporating mechanisms such as, the Medicines Patent Pool, Compulsory Licensing and Access to Medicines Index, it can facilitate increased accessibility of affordable medicines. Adopting the flexibilities allocated by the World Trade Organisation will enable to achieve the universal goal of healthy lives for all and lead to Global Sustainable Development.
Submission
Eradication of extreme poverty and discrimination is the key to progressive realisation of sustainable development with an empowering impact on the population’s health (UN, 2015). The Constitution of World Health Organization (WHO), the Universal Declaration of Human Rights, the International Covenant on Economic, Social and Cultural Rights (ICESCR) and the Alma-Ata Declaration of 1978 (WHO, 1978) are some of the leading evidence based principles that make it obligatory for Member States to take deliberate and concrete steps for full realization of the Right to Health, with emphasis on vulnerable and marginalized groups (WHO et al., 2012). The realization of right to health gets adversely affected due to high prices of essential medicines and lack of equity in their, and need for out-of-pocket payments by the poor and vulnerable (WHO et al., 2012). Hence the Millennium Development Goals (MDG) 8.E and now the Global Sustainable Development Goal (SDG) 3.8 have continued to focus on achieving universal health coverage, including affordable essential medicines and vaccines for all (UN, 2015). Though, health is constitutionally recognised as a fundamental Human Right in 135 nations, only four national constitutions specifically mention universal access to medicines (Perehudoff et al., 2010). The results of an interim assessment of the implementation of the WTO’s Medium-term strategic plan 2008–2013 to achieve a set of health goals, indicates that, of the 11 strategic objectives, progress achieved was maximum under strategic objective 1 for communicable diseases while the least under strategic objective 3 for non-communicable diseases (WHO, 2011; Mendis et al., 2012).
The present paper analyses access to essential medicines, especially for those related to the treatment of non-communicable diseases (NCDs) in light of the Right to Health and the Trade Related Intellectual Property Rights (TRIPS) of the WTO in Low and Middle Income Countries (LMICs), with a special focus on South Africa.
The first section reviews the provisions of TRIPS and their impact on access to essential medicines in LMICs. The second section focuses on the access to essential medicines in South Africa in light of IPRs, while the third section highlights future course of action needed in patent laws to make the essential medicines for NCDs both affordable and accessible in LMICs.
TRIPS and Essential Medicines
Many studies have highlighted the inaccessibility to affordable essential medicines in public sector health care facilities especially to poor sections of societies in LMICs (Jung and Kwon, 2015; Mendis et al., 2012; WHO 2010a, 2010b). This forces patients to purchase expensive medicines from the private sector, or forgo treatment (WHO, 2010b). Medicine product patents introduced by the WTO and TRIPS has resulted in monopoly control by the patent holders and delayed entry of lower-cost generic medicines (Jung and Kwon, 2015; WHO and HAI, 2011) leading to exacerbated poor access to essential medicines in LMICs (Jung and Kwon, 2015; Watal, 2000).
Income inequalities produce highly convex demand curves for essential medicines- a normal situation in many developing countries associated with profit-maximizing strategies (Sean Flynn et al., 2009). Several policy and implementation incoherencies such as TRIPS-plus agenda of evergreening and data exclusivity have a negative impact on advancing human rights and achieving sustainable health outcomes (Quigley, 2015). Institutional leaderships have allowed sustainable policy impact in adopting mechanisms such as Medicines Patent Pool (MPP) that enables building partnerships under creative licensing strategies to accelerate medical innovation (Krattiger, 2013). Apart from encouraging generic manufacture and the development of new formulations benefiting low-cost medicines to LMIC, the development of poly pills advances sustainable health outcomes (MPP, 2016; UNITAID, 2016).
Non-Communicable Diseases: The rapidly growing NCDs epidemic is a major cause of global morbidity and mortality. NCDs accounted for 68% of global deaths, of which 80% occurred in LMICs, with over 40% of these being premature deaths under the age of 70 years (WHO, 2014). The epidemic is predicted to cause approximately 75% of all global deaths by 2030 (WHO, 2015). NCDs are a great danger to sustainable human development, as they result in an increased dependency ratio due to disability and the loss of labour, exorbitant continuous treatment costs, and, thereby, lead to a vicious circle of poverty among the poor (WHO, 2013). Hence, there is a vital need for access to appropriate medicines for treating NCDs (Abegunde et al., 2007).The WHO’s Global Action Plan includes a voluntary target of 80% availability and affordability of essential medicines for the prevention and treatment of diabetes, cardiovascular disease, and respiratory diseases, both in public and private health facilities (WHO, 2016). However, access to medicines and vaccines to prevent and treat NCDs is unacceptably low, globally and, more especially, in LMICs (Abegunde et al., 2007; Hans V. Hogerzeil et al., 2013; WHO and HAI, 2011).Various case studies in LMICs, pertaining to NCDs in general and specific ailments have revealed the lack of availability of essential medicines, especially in public health facilities leading to adverse economic consequences (Beran et al., 2015; Jingi et al., 2014; Katende et al., 2015; Robertson et al., 2015). High Price and variations across countries for essential medicines such as insulin results in various incoherence issues ultimately leading to deprivation of affordable medicines for poor in LMICs (HAI, 2016).Valuable experiences (Kishore et al., 2011) gathered from thelong sustained HIV AIDS programs can be used to identify the way forward to manage the challenges in treating the NCDs (Hans V. Hogerzeil et al., 2013).
Access to Essential Medicines in South Africa
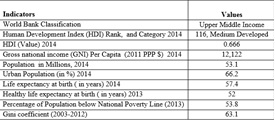
South Africa, an Upper Middle Income Country with a medium human development index, faces a very high incidence of poverty and income inequality (Table 1). The Constitution of South Africa has included the Right to Health as a Human Right and health objectives of the country guarantee the availability, and the accessibility of essential medicines to all of its citizens (Government of South Africa, 1996). However, its Constitution does not specifically commit to access to essential medicines (WHO, 2010b) but describes access to health care, goods and services in more general terms (Perehudoff et al., 2010).The National Drug Policy (NDP) for South Africa, 1996 aims to provide equal access to medicines for all through the Essential Drugs Programme (DOH, SA, 2016).
Table 1: Development Indicators of South Africa
Source: Compiled from data of The World Bank 2015; UNDP 2015
The country faces a double burden of both communicable and non-communicable diseases, leading to low life expectancy (Table 2).
Table 2: Proportional Mortality by Causes in South Africa
Source: World Health Organization, 2014a.
The proportion of non-communicable diseases, though lower than when compared to that in advanced and many developing countries, due to higher death rates on account of communicable diseases, is remarkably high, when consideringabsolute numbers due to NCD deaths (Table 3). To make things worse, the toll of NCDs falls mainly on the productive population.
Table 3: Age-standardised Death Rate due to NCDs by Type per 100,000 in South Africa (2012)
Source: World Health Organization, 2014b.
Healthcare delivery in South Africa is characterized by a two-tier system of private and public healthcare providers. Until the process of democratisation and universal franchise, the private healthcare was funded by medical schemes, which covered up to 20% of the country’s population, consisting mostly the white section of the population, while the public sector,struggling with poor conditions, catered to the rest of the population (NDP, 1996). The decreasing life expectancy might have proven a cause for concern for health services, resulting in an increase in per capita health expenditure (Table 4) and leading to a nearly twofold increase in per capita health expenditure between 2000 and 2005. This has resulted in a slow increase in life expectancy. The share of the public sector has also been increasing since 2005, although the private sector still makes a higher contribution. This clearly highlights the need for efforts to increase the accessibility of healthcare in a society with a wide income inequality,and high levels of poverty.
TABLE 4: HEALTH EXPENDITURE AND LIFE EXPECTANCY IN SOUTH AFRICA
Source: WHO data base
Medicine prices: Even though fast escalation of the prices of medicines is a global phenomenon, private sector medicine prices in South Africa are among the highest in the world. Considering this, the Government of South Africa has developed a National Drug Policy, consisting of the development of an essential drugs programme for public sector primary healthcare facilities (HST). Based on the primary healthcare concept of universal access to healthcare, the essential drugs programme will ensure that drugs for treating the most common ailments are made available, and accessible to the entire population, at an affordable cost to both the government and to consumers (HST).
The economic objective of the National Drug Policy was to lower the cost of medicines in both the private and public sectors, and to promote the cost-effectiveness and the rational use of medicines (NDP for SA). The weak Rand has affected importers of finished medicines and domestic manufacturers using imported raw ingredients by pushing up the cost of production, and thus reducing profit margins. To mitigate the increase in cost, the Health Department of South Africa is planning to allow pharmaceutical companies an additional price increase. However, the step of offering relief to drug companies under margin pressure will adversely affect medical schemes and consumers with further rises in medicine prices. Medicine prices in the private sector are tightly regulated by the Health Department, usually only allowing a one-time annual price increase, based on a weighted formula that includes the consumer price index (70%) and the exchange rate (30%) (Bdlive, 2016). Even then, the prices of medicines in South Africa are on par with some of the highest in the world, making treatments unaffordable to the majority of people, due to limited know-how and a lack of manufacturing capacity by local producers (MSF Access Campaign, 2013). Although the South African medicine pricing system is based on a single exit price, which is in turn based on the manufacturer price decided by the Department of Health, the access to essential medicines for the average South African is limited. It has been observed that some South African based patented medicines, like the Cancer drug Imatinib, fix a higher price in South Africa compared to their pricing in other countries (TAC, MSF and Research and Information System, 2013).
Patent Rights in South Africa
Activist communities believe that patents are barriers to the accessibility to affordable medicines in many developing countries, while the pharmaceutical industry argues that theyare necessary to assure future research, development, and the commercial viability of the industry (Attaran A, 2013). The South African Patents Office operates a formal or depository patent registration system, which is one of the cheapest systems in the world (Pouris and Pouris, 2011). In South Africa, highest proportion of patents applications were granted patents followed by Russia, while the number granted was very low in India, Brazil and Chinaamong BRICS countries in 2011 (Table 5). However, in absolute numbers, China granted the most patents, followed by Russia. All of the BRICS countries follow examination system, with the exception of South Africa (Yu-Fang Wen and Thapi Matsaneng, 2014).
Table 5: Patent applications and grants in BRICS members’ Patent Offices, 2011
Source: WIPO: World Intellectual Property Indicators 2012
In South Africa, the residents’ patent applications and grants formed only around 10% of the total (Table 6). As the South African patent system does not investigate the merits of each patent based on experiment, multiple patents for a single medicine could easily be granted (TAC, MSF and Research and Information System, 2013) for a 20 year period, and, furthermore, if a secondary patent or follow-up patents (“ever-greening”) are granted, competition would be restricted (European Medicines Association, 2008). The depository patent system probably allows for unjustifiable patents that might restrict competition by discouraging research and development by competitors, and would restrict access by generic manufactures. Substantive patent examination systems could eliminatelow-quality patents that protect inventions of limited novelty, or from providing overly broad protection.
Table 6: Patent applications and grants by the South African Patent Office, 2011
Source: WIPO: World Intellectual Property Indicators 2012
Competition Policy:Competition policy plays an important role in ensuring that there is sufficient competition amongst manufacturers in making pharmaceuticals more accessible and affordable which can be achieved by competition between manufacturers of different originator medicines and/or competition between originator and generic manufacturers (Yu-Fang Wen and ThapiMatsaneng 2014).
Patent Pool: A pill with a combination of multiple medicines will help treating patients under scarcity of resources. However, this can be obstructed due to patents on individual medicines. The Medicines Patent Pool is suggested as a solution to this problem, and is expected to stimulate competition. This can reduce the uncertainty for generics companies in obtaining the rightsto produce a particular medicine from several patent holders, and can be beneficial to all stakeholdersbymaking these medicines accessible in developing countries (UNITAID).
One of the main arguments put forward by industry with respect to the need for strict protection of IPRs is the high cost of R&D for new medical products. However, it has been observed that expenses incurred on pharmaceutical R&D by the private sector formed less than 8 per cent of global yearly medicine (and other medical tools) sales in 2010 (Love, 2011). American pharmaceutical manufacturers spent almost double their R&D expenses on marketing (Gagnon & Lexchin 2008), and generally take home more in profit than they spend on R&D annually (Tomlinson & Rutter 2014). R&D expenses are not solely borne by pharmaceutical companies, as they are also financed through public funds generated from tax contributions by the general public and other sources (Røttingen, 2013). In South Africa, the Government was the chief funder, contributing 44.4% of the total R&D expensesduring 2009-10 (DST, 2013).
Corporate Social Responsibility:The corporate sector has a responsibility towards the general public,in addition to its responsibility to earn a profit for its shareholders. However, the profit-oriented corporate sector turns a blind eye towards this responsibility to the consumers and general public. This can be clearly observed in the statement of the CEO of Bayer, announcingthat its cancer drug was not developed for poor patients in India, but “for western patients who can afford it,” in response to India’s issuance of a compulsory licence on Sorafenib, allowing generic manufacturing of the medicine in the country (Gokhale, 2014). This medicine, patented in South Africa, is not available to patients in the public sector of the country due to its high price, indicating that development in the pharmaceutical industry is predominantly influenced by the market, rather than the health needs of society ((Tomlinson & Rutter, 2014 ).In South Africa, like in many other developing countries, there is little evidence to support the claim of the industry that the adoption of public health safeguards would weaken the development of future medicines.Hence, campaigns like ‘Fix the Patent Laws’ advocate for a more rational patent regime by considering health, developmental, social, and economic needs of the country, along with relevant evidence, rather than a purely capitalist ideology (Tomlinson & Rutter, 2014).
Draft New Intellectual Property (IP) Policy of South Africa: The draft New Intellectual Property (IP) Policy of South Africarecommendsanamendment of legislation to incorporate the Doha Declaration “flexibilities” and incentive schemes in the areas of IP to achieve developmental goals of poverty alleviation and health.It argues for the necessityof applying competition law to patent lawsunder over-concentration, dominance, or abuse by IP holders. It recommends the use of compulsory licences, and parallel importation,for making medicines affordable, and balancing trade withhealth interests in patent protection. Considering the resource constraintsin the country, it recommends a combination of the depository and examination systems for the registration of patents.
India, while obliging to recognise pharmaceutical product patents in terms of the TRIPs, incorporated a number of important flexibilities, such as compulsory licencing, Parallel Imports, the Bolar Exception in its Patents (Amendment) Act 2005, in order to facilitate access to affordable medicines (Health Review). It has utilised section 3(d) of its Patents Act,which relates to higher standards for patentability, to deny Novartis a patent on the anti-leukaemia drug Imatinib (sold as Glivec). It granted a compulsory licence for a generic version of Bayer’s anti-cancer medicine Sorafenib (sold as Nexavar), on the basis that the local generic manufacturer Natco Pharma was able to supply the drug at 3% of Bayer’s price, which was exorbitant (WIPO 2014). These indicate how developing countries can utilise TRIPs provisions to make essential medicines affordable to their populations.
Way forward
Using SDG 3 as a pivotal point, it is essential to drive the process of promoting affordability of medicines for NCDs, by using a public health approach and by utilizing the flexibilities of TRIPs. The short term gains of the corporate sector act as a hindrance to achieving the universal goal of a healthy life for all and of sustainable development. The WTO, which advocates for free trade, and for the elimination of restrictions and monopoly power, requires a review of TRIPs in light of the broader goals of health, to facilitate sustainable development globally. In the era of globalisation, developed member states of WTO should place global human interests ahead of the financial interests of their corporate sectors.
Bibliography and References
Abegunde DO, Mathers CD, Adam T, Ortegon M, Strong K. The Burden and Costs of Chronic Diseases in Low-Income and Middle-Income Countries. The Lancet. 2007 Dec 14;370(9603):1929–38.
Anatole Krattiger (2013), Promoting Access to Medical Innovation Global Challenges Division, WIPO September 2013 http://www.wipo.int/wipo_magazine/en/2013/05/article_0002.html
Attaran A, How Do Patents And Economic Policies Affect Access To Essential Medicines In Developing Countries? Health Affairs vol 23(3) July 5, 2013, p 159. http://content.healthaffairs.org/
Banerjee, A. 2006. Who has responsibility for access to essential medical drugs in the developing world? Ethics and Economics, 4 (2), 2006, University of Oxford, UK
BDlive, 2016Healthcare inquiry could lead to further scrutiny, 15 January 2016: http://www.bdlive.co.za/national/health/2016/01/19/extra-price-hike-on-cards-for-medicine
Business Week. Available at: http://www.businessweek.com/news/2014-01-21/merck-tobristol-myers-face-more-threats-on-india-drug-patents#p2 cited in Fix the Patent Laws.
Declaration of Alma-Ata, International Conference on Primary Health Care, Alma-Ata, USSR, 6-12, September 1978.
DST. (2013). National Survey of Research and Experimental Development (2009/10 Fiscal Year). https://www.google.co.za/search?q=DST.+(2013).+National+Survey+of+Research+and+Experimental+Development+(2009%2F10+Fiscal+Year).&oq=DST.+(2013).+National+Survey+of+Research+and+Experimental+Development+(2009%2F10+Fiscal+Year).&aqs=chrome..69i57.2053j0j7&sourceid=chrome&es_sm=93&ie=UTF-8
Ecology International. Available at: http://keionline.org/node/1152
European Medicines Association, Patent-related barriers to market entry for generic medicines the European Union: A review of weakness in the current European system and their impact on market access of generic medicines, May 2008. http://www.ieis.org.tr/ieis/assets/media/EGA%20-%20IP_Barriers_web.pdf
Gagnon M-A, Lexchin J (2008) The Cost of Pushing Pills: A New Estimate of Pharmaceutical Promotion Expenditures in the United States. PLoS Med 5(1): e1. doi:10.1371/journal.pmed.0050001
Geneva, November 3 to 7, 2014Study on the role of patent systems in promoting innovative medicines, and in fostering the technology transfer necessary to make generic and patented medicines available in developing countries and least developed countries , Document prepared by the Secretariat www.wipo.int/edocs/mdocs/scp/en/scp_21/scp_21_8.pdf
Gokhale. (2014). “Merck to Bristol-Myers Face Threats on India Patents (Correct)”. Bloomberg
Health Policy and Legislation 1 SAHR 2012/13 (Health Review)
Health Systems Trust http://www.hst.org.za/publications/essential-drugs-list-programmeeditorial
http://ip-unit.org/wp-content/uploads/2013/09/DRAFT-IP-POLICY.pdf
Hogerzeil HV, 2013 Lancet. 2013 Feb 23;381(9867):680-9. doi: 10.1016/S0140-6736(12)62128-X. Epub 2013 Feb 12. http://www.thelancet.com/journals/lancet/article/PIIS0140-6736(13)61046
Hogerzeil HV1, Liberman J, Wirtz VJ, Kishore SP, Selvaraj S, Kiddell-Monroe R, Mwangi-Powell FN, von Schoen-Angerer T; Lancet NCD Action Group. http://www.ncbi.nlm.nih.gov/pubmed/23410612
http://ip-unit.org/wp-content/uploads/2013/09/DRAFT-IP-POLICY.pdf
http://www.fixthepatentlaws.org/wp-content/uploads/2013/10/S27-TAC-MSF-Submission_on_IP_Policy.pdf
http://www.hst.org.za/news/africa-needs-access-affordable-medicine
Kishore SP, Vedanthan R, Fuster V. Promoting Global Cardiovascular Health: Ensuring Access to Essential Cardiovascular Medicines in Low- and Middle-Income Countries. J Am Coll Cardiol. 2011;57(20):1980-1987. doi:10.1016/j.jacc.2010.12.029. http://content.onlinejacc.org/article.aspx?articleid=1146488
Love. J. (2011). ‘Pharmaceutical global R&D was 7.9 per cent of sales in 2010’. Knowledge May 2011
Mendis S, Al Bashir I, Dissanayake L, Varghese C,etal., Gaps in capacity in primary care in low-resource settings for implementation of essential non-communicable disease interventions.International Journal of Hypertension, Volume 2012, Article ID 584041, 7 pages
MSF Access Campaign. South Africa’s IP Policy. Way Forward? Benefits of Substantive Examination for Pharmaceutical Patent Claims, IP Forum, South Africa, 2013.
National Drug Policy (1996), Government of South Africa.
Pouris A, Pouris A. Patents and economic development in South Africa: Managing intellectual property rights. S Afr J Sci. 2011;107(11/12), Art. #355, 10 pages. http:// dx.doi.org/10.4102/sajs. v107i11/12.355http://www.sajs.co.za/sites/default/files/publications/pdf/355-6888-1-PB.pdf
Promotion Expenditures in the United States. PLoS Med 5(1): e1.
Promotion of access to essential medicines for non-communicable diseases: practical implications of the UN political declaration.
Quigley, F. The Trans-Pacific Partnership and Access to Medicines, Perspectives, June 18, 2015.
Review Series on Pharmaceutical Pricing Policies and Interventions
Røttingen J-A, 2013 Røttingen J-A, Regmi S, Eide M, Young M, Viergever R, Årdal C, Guzman J, Edwards D, Matlin S, Terry R. (2013) Mapping of available health research and development data: what's there,
Sean Flynn, Aidan Hollis, & Mike Palmedo, January 2009, University of Calgary, Department ofEconomics.
TAC, MSF and Research and Information System, Why South Africa should examine pharmaceutical patents, Briefing January 2013, p 4. WHY SOUTH AFRICA SHOULD EXAMINE PHARMACEUTICAL PATENTS How legislative reform could boost the affordability and accessibility of medicines for South AfricansBriefing by TAC, MSF and RIS – January 2013http://www.ris.org.in/sites/default/files/pdf/Policy%20Brief-60.pdf
Tomlinson C. & Rutter L. (Updated version February 2014) The Economic & Social Case for Patent Law Reform in South Africa, RESEARCH PAPER www.fixthepatentlaws.org@FixPatentLawTretment Action Plan
UNa: United Nations: http://www.un.org/en/index.html
UNb: United Nations: Universal Declaration of Human Rights http://www.ohchr.org/EN/UDHR/Documents/UDHR_Translations/eng.pdf
UN: http://www.un.org/sustainabledevelopment/sustainable-development-goals/
UNITAID The Medicines Patent Pool Stimulating Innovation, Improving Access, UNITAID : An Important Partner
Watal, J. (2000). Access to Essential Medicines in Developing Countries: Does the WTO TRIPS Agreement Hinder It? Science, Technology and Innovation Discussion Paper No.8 ,Center for International Development, Harvard University, Cambridge, MA, USA.
what's missing, and what role is there for a global observatory? Available at
WHO“Access, Quality and Rational Use of Medicines and Essential Drugs” at www.who.int/medicines/edm-concept.html
WHO http://apps.who.int/nha/database
WHO Library Cataloguing in Publication Data, Regional strategy for improving access to essential medicines in the Western Pacific Region, 2005 -2010, World Health Organization 2005.
WHO, “Access, Quality and Rational Use of Medicines and Essential Drugs” at www.who.int/medicines/edm-concept.html
WHO, Global Status Report on Non-Communicable Diseases, 2014[Internet]. [cited 2015 May 11]. Available from: http://apps.who.int/iris/bitstream/10665/148114/1/9789241564854_eng.pdf?ua=1
WHO. (2014a). Noncommunicable Diseases (NCD) Country Profiles, 2014. http://www.who.int/nmh/countries/zaf_en.pdf.
WHO. (2014b). Global status report on noncommunicable diseases 2014. Switzerland. Retrieved from http://www.who.int/nmh/publications/ncd-status-report-2014/en/
WHO. Global Health Expenditure Database [Internet]. 2014 [cited 2015 Aug 20]. Available from: http://apps.who.int/nha/database/Select/Indicators/en
WHO. (2015). Non-Communicable Diseases Factsheet [Internet]. 2015 [cited 2015 Aug 20]. Available from: http://www.who.int/mediacentre/factsheets/fs355/en/
WHO/HAI (2011) Working Paper 4: Competition Policy Project on Medicine Prices and Availability
WIPO (2014), Standing Committee on the Law of Patents, Twenty-First Session
WIPO (2012), World Intellectual Property Indicators 2012, Cited by Yu-Fang Wen and ThapiMatsaneng 2014
Yu-Fang Wen and Thapi Matsaneng (2014), Patents, Pharmaceuticals and Competition: Benefiting from an effective patent examination system. http://www.compcom.co.za/wp-content/uploads/2014/09/Patents-Pharmaceuticals-and-Competition
http://www.fixthepatentlaws.org/wp-content/uploads/2013/10/S27-TAC-MSF- Submission_on_IP_Policy.pdf