Lead Author: Prerna Mingma Bomzan
Additional Authors: Sanya Reid Smith and Mirza Alas Portillo
Organization: LDC Watch
Country: Nepal; Switzerland
***This contribution is submitted by LDC Watch a global alliance of civil society organisations (CSOs), networks and movements based in the LDCs (www www.ldcwatch.org); and supported by Center for Health Human Rights & Development (www.cehurd.org), The Health GAP (healthgap.org), Third World Network (www.twn.my) and Yolse Santé Publique & Innovation
Abstract
This Submission concerns achievement of the Right to Health in Least Developed Countries (LDCs) by promoting access to affordable pharmaceutical products, developing a technological base including pharmaceutical production capacity and transfer of technology for that purpose. This submission recommends inter alia
- Unconditional extension of the general transition period, which will expire on 1 July 2021 for as long as they remain a LDC;
- Implementation of paragraph 18 of the 2012 WTO Accession Guidelines that requires Special and Differential Treatment provisions of the WTO (i.e. the transition periods) to be applicable to all acceding LDCs;
- WTO Members to refrain from imposing TRIPS plus conditions on acceding LDCs.
- For LDCs to fully utilize the General TRIPS Transition Period and the Specific Pharmaceutical Transition Period and Other TRIPS flexibilities as well as avoid TRIPS-plus measures.
- Reform of the “Harare Protocol” of the African Regional IP Organization (ARIPO) to incorporate the LDC transition periods, adopt rigorous patentability standards and administrative pre- and postgrant opposition procedures.
- Review the Bangui Agreement and ensure that it fully and optimally incorporates the transition periods and the full range of TRIPS flexibilities available and avoids TRIPS plus provisions.
- An independent review of technical assistance being provided by WIPO and other agencies to LDCs to assess its suitability for LDCs, particularly from a development and public health perspective.
- Institute an independent monitoring, evaluation and accountability mechanisms with regard to technical assistance provided to LDCs.
- Implement measures to operationalize Article 66.2 of TRIPS Agreement
- The United Nations Technology Bank for LDCs should review its approach to IP and amend it to bring it in line with the achievement of right to health in LDCs.
Submission
Least developed countries (LDCs) represent the poorest countries in the world.1 There are currently 48 countries2 designated by the UN3, 34 are members of the WTO.
LDCs are characterized by low per capita income, low level of human development, and economic vulnerability, are at bottom of technology development4 and challenged by natural calamities (e.g. Haiti and Nepal), violence and political instability, symptomatic of poverty, inequalities and social injustices.5
LDCs face significant health burdens related to communicable6 as well as non-communicable diseases (NCD).7
Health expenses in these countries are usually borne out-of pocket. Donor funding is limited to specific disease areas (e.g. HIV, TB, Malaria) and is reducing. As countries graduate from the LDC status health financing will be negatively impacted, and the strain on national health budgets will grow. Thus availability of affordable pharmaceutical products is imperative if the health needs of LDCs are to be addressed.
Access to pharmaceutical products is considered integral to the right to the highest attainable standard of health. Its integral role in achieving Universal Healthcare (UHC) and the commitment of governments to use to the full TRIPS flexibilities is noted in multiple UN resolutions and processes8 . The UNGA SDG resolution recognizes explicitly the link between treatment access and the use of TRIPS flexibilities in Goal 3.b.
Against this background, the most important policy options available to LDCs to facilitate affordable access are the transition periods, which exempts LDCs from TRIPS implementation. They are also fundamental to the development of a viable technological base including pharmaceutical production capacity. Moreover, extensive IP rights are negatively connected to their development interests.9
Despite its importance, utilization of the transition periods is inadequate, thus impacting the fulfillment of the right to health. This due to several factors but in particular technical assistance provided to LDCs by WIPO10 and other agencies (US, EU, Japan)11, regional IP systems12, the need to repeatedly request extensions of transition periods. These are also reasons why LDCs’ have yet to fully and properly incorporate other TRIPS flexibilities into their national patent laws.
LDC Transition Periods
Article 66.1 of TRIPS recognizes “the special needs and requirements of least-developed country Members, their economic, financial and administrative constraints, and their need for flexibility to create a viable technological base” and accordingly grants LDCs a transition period, which may be extended by submitting a “duly motivated request”. The preamble of TRIPS reinforces this flexibility.13
At present LDCs enjoy a general transition period until 1st July 2021 during which period LDCs do not have to implement the TRIPS provisions except for Articles 3, 4 and 5 of the TRIPS Agreement.14 LDCs also enjoy a specific pharmaceutical transition period until 1 January 2033, wherein, LDCs do not have to grant or enforce pharmaceutical patents and test data protection. 15 Further they have been granted a waiver from mailbox and provide exclusive marketing rights obligations during the transition period.16 These transition periods may be extended by LDCs on making a request to the WTO TRIPS Council (Article 66.1).17 It is clear from the text of Article 66.1 that LDCs’ request is non-negotiable.
It is of note however that LDCs’ requests for extensions of transition period sought duration of as long as a country is a LDC18 and although its requests obtained widespread support from UN agencies, civil society, developed countries opposed the requested duration eventually forcing through time limited transition periods, thus undermining LDCs rights under Article 66.1.19
Utilization of General TRIPS Transition Period
The general transition period allows LDCs full flexibility to not implement the TRIPS Agreement until 1 July 2021 and beyond (if extended).
Most LDCs inherited their current patent regimes through colonial rule or adopted maximalist IP provisions recommended by WIPO. A review of LDCs’ patent laws shows that despite the availability of transition periods, some LDCs’ patent laws are TRIPS compliant. Those not fully compliant with TRIPS still accords high patent protection and several appear to be considering bills for early TRIPS compliance20. Of the LDCs still negotiating their agreements for accession to the WTO, Bhutan and Sao Tome and Principe appear to have TRIPS compliant patent laws in place from as far back as 2001. A majority of LDCs, including non-WTO members have joined the Patent Cooperation Treaty21 though there is no requirement to do so even under TRIPS.
Two regional IP groupings in Africa have expedited TRIPS and TRIPS-plus compliance for a large number of African LDCs.
For members of the African Intellectual Property Organization (OAPI), TRIPS-plus compliance has come through the “Bangui Agreement”. OAPI is made up of 17 Francophone countries, of which 14 are LDCs. The Bangui Agreement covers various IP matters including patents. It centralizes the filing of applications and grant of patents, as well as prescribes on post grant matters and enforcement. Effectively the Agreement supersedes national laws.
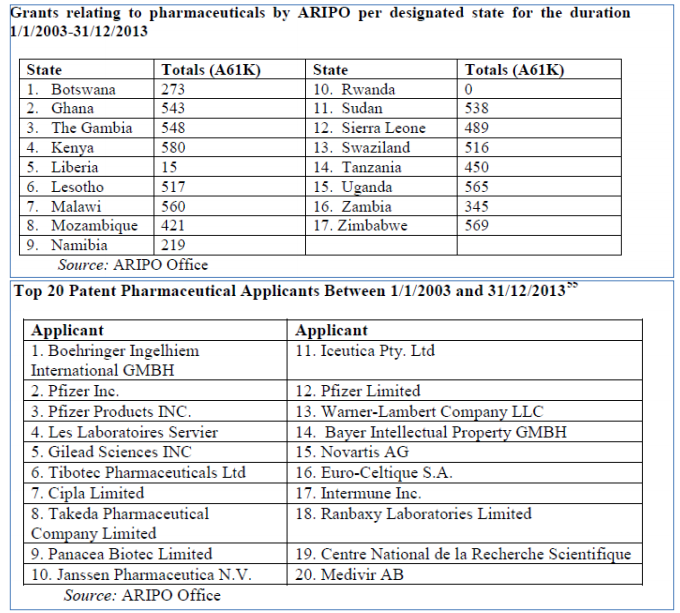
Another regional IP grouping, the African Regional Industrial Property Organization (ARIPO) is limited to the examination and grant of patents leaving other aspects to national laws. ARIPO is made up of 19 anglophone countries of which 18 are members of the “Harare Protocol” (of this 12 are LDCs (3 are nonWTO members)), which governs industrial property.
ARIPO's Harare Protocol allows some flexibility for its members, which may, within 6 months, notify that a patent granted at ARIPO would not have effect in that particular country. However, most ARIPO 4 members do not actually take advantage of this rule resulting in hundreds of pharmaceutical patents being granted.
It is apparent the utilization of general transition period is inadequate. Transition period was granted in view of the special needs and vulnerabilities of LDCs and their need for maximum policy space to develop technological capacity. The history of IP and development confirms that premature implementation of TRIPS compliant patent regime hinders development prospects as it prevents “reverse engineering” and access to tools and technologies needed to develop a technological capacity including pharmaceutical production capacity.22 LDCs simply do not have the national conditions to be able to benefit from such a regime such as market power, highly skilled personnel, solid technological base and infrastructure, government investment in R&D etc.
Utilization of Pharmaceutical Transition Period 5
In the case of the pharmaceutical transition period some LDCs have taken steps to incorporate the same into their domestic patent law (e.g. Cambodia, Burundi, Madagascar, Uganda, Rwanda). 23.
However some of these countries are members of ARIPO (Burundi, Uganda, Rwanda), which has yet to implement a pharmaceutical transition period at the regional level. Consequently patents granted extend to their territories.24 25 In short policy incoherence between national and regional laws is undermining effective use of the pharmaceutical transition period and fulfillment of the right to health.
In the case of OAPI, it recently amended the Bangui Agreement. Article 46 now reads: Member States that are LDCs are not obliged to implement the provisions of Annex I regarding patents consisting of, or related to, a pharmaceutical product, nor to implement the provisions of Annex VIII regarding confidential information, until 2033 or the date on which they stop to be classified as an LDC. It is hoped that with this provision, pharmaceutical patents granted by OAPI will not apply to LDC members of OAPI. Failure to effectively operationalize this provision will affect the right to health.
Variable incorporation and use of TRIPS flexibilities by LDCs
There are multiple flexibilities in the TRIPS Agreement that could be used for access to affordable generic pharmaceutical products.
A review of LDC laws shows that flexibilities have been incorporated in a variety of ways, often by not optimizing the policy space available to them. For instance, pre-grant opposition procedures feature in the patent laws of Burundi, Zanzibar and Uganda. However some of the conditions in these laws may create barriers for public interest groups to file an opposition. It is recommended that LDCs should have strict patentability standards to avoid frivolous patents and patent evergreening.26 Even so few LDCs have adopted specific provisions to prevent evergreening. 27 Even where provisions are incorporated in national patent law, in practice they are not utilized. Most LDCs do not undertake substantive patent examination, and often rely on regional patent offices (ARIPO/OAPI) or foreign patent offices (e.g. the European Patent Office, the Japan Patent Office etc.) for examination of patents, which in turn facilitates patent evergreening.28 In fact the regional patent offices have been criticized for failing to incorporate key pre-grant flexibilities that are important to promote affordable access (such as pre-grant opposition).29
Once a patent has been granted, there are policy options available to address adverse effects on access to pharmaceutical products or to research and development (e.g. bolar and research exceptions, compulsory licenses, parallel importation etc.). However, these are not universally incorporated in all the patent laws in LDCs.30 The laws of all LDCs do provide non-voluntary licenses, albeit under different conditions and through different procedures, in some cases restrictive and burdensome. Ensuring that the grounds for issuing CL are broad, the procedures simple to use, are not subject to injunctions and other procedural restrictions will require a review of these provisions.
In Asia, Myanmar, Cambodia and Laos are members of the Association of Southeast Asian Nations (ASEAN) which is rapidly moving towards IP harmonisation that so far has concentrated on putting in place higher IP protection than on the use and incorporation of TRIPS flexibilities.31
Another area of concern is anti-counterfeit legislation reportedly being considered by the East African Community (EAC), Uganda, Tanzania, Zambia and Malawi that extends to patents and prescribes TRIPS- 6 plus IP enforcement measures, which will harm rather than protect public health. 32 Criminal penalties for patent infringement which features in a majority of the laws of LDCs is also a huge concern33.
TRIPS-plus IP enforcement initiatives adversely impacts access to affordable pharmaceutical products as well as local production, as it deter or creates a chilling effect with regard to, production, import or export of pharmaceutical products.
TRIPS-plus measures in LDCs: WTO Accession and FTAs
Since the WTO was established, nine LDCs have negotiated entry into the WTO.34 Although the health safeguards discussed above should be available to all LDC members of the WTO, LDCs that negotiate accession to the WTO after it was established have often faced demands by developed countries to forego some of these rights35 inconsistent with achievement of the right to health.
Paragraph 18 of the 2012 Accession Guidelines explicitly reaffirms “that the Special and Differential Treatment, as set out in the Multilateral Trade Agreements, Ministerial Decisions, and other relevant WTO legal instruments, shall be applicable to all acceding LDCs from the date of entry into force of their respective Protocols of Accession.” 36 This means that developed countries demands aimed at nullifying the use of the transition period are illegimate. Paragraph 18 confirms that acceding LDCs may utilize transition periods available to other LDCs which are members of the WTO.
The inclusion of LDCs in free trade agreement negotiations such as Cambodia, Myanmar and Lao PDR in the Regional Comprehensive Economic Partnership (RCEP) negotiations or of many African LDCs in economic partnership agreement negotiations with the European Union is also of concern as negotiations involving developed countries are likely to include limitations on the ability of LDCs to make full use of the transition periods or fully incorporate public health safeguards in their laws.
Technology Transfer
Article 66.2 of the TRIPS Agreement requires developed countries “to provide incentives to enterprises and institutions in their territories” to promote technology transfer to LDCs. The importance of technology transfer is underscored in the Doha Declaration37. However thus far there has not been any meaningful transfer of technology to LDCs.38
Developing local or regional pharmaceutical production capacity is a fundamental aspect of access to pharmaceutical products and thus imperative to the fulfillment of the right to health. There is growing concerns of the impact of the overwhelming reliance on pharmaceutical imports on affordability, availability and long term sustainability.39 Multiple African LDCs are covered by a plethora of regional pharmaceutical manufacturing plans40 including the African Union's Pharmaceutical Manufacturing Plan for Africa Business Plan (PMPA) 2012 which "is based on the belief that industrial development and the development of the pharmaceutical sector is not in conflict with public health imperatives and that the industry should in fact be developed with the long term aim of promoting access to quality essential medicines."41
RECOMMENDATIONS
i. For WTO Members
- The LDC general transition period, which expires on 1 July 2021, should be extended for as long as a country remains a LDC and not be subject to any conditions. 7
- Implement paragraph 18 of the 2012 WTO Accession Guidelines that states “the Special and Differential Treatment, as set out in the Multilateral Trade Agreements, Ministerial Decisions, and other relevant WTO legal instruments, shall be applicable to all acceding LDCs from the date of entry into force of their respective Protocols of Accession.”
- Ensure that acceding LDCs fully enjoy and utilize the transition periods Refrain from imposing TRIPS plus conditions on acceding LDCs.
- Implement measures to operationalize Article 66.2 of the TRIPS Agreement42
ii. For Least Developed Countries:
- Fully utilize the General TRIPS Transition Period and the Specific Pharmaceutical Transition Period.
- Review national intellectual property laws especially the patent law to ensure that they fully and optimally incorporate the transition periods as well as the TRIPS flexibilities available to LDCs.
- Seek pro-development and pro-public health technical assistance.
- Undertake transparent public consultation that includes civil society concerned with affordable access when amending or formulating patent laws.
- Make full use of public health safeguards incorporated in national laws
- Reject TRIPS-plus requirements including in WTO accession or FTA negotiations.
ii. For Regional IP Systems and Initiatives
- ARIPO’s Harare Protocol should exempt the territory of LDCs from the grant of any pharmaceutical patents.
- ARIPO should reduce reliance on foreign examination systems and adopt rigorous patentability standards with regard to examining of pharmaceutical applications, paralleling those adopted by Argentina43 to avoid patent evergreening.
- ARIPO should increase its fees related to the filing and examination of applications and maintenance of patents to avoid proliferation of frivolous patents.
- The Harare Protocol should establish administrative pre- and post-grant opposition procedures, to enable any person to file a notice of opposition before the ARIPO Office.
- OAPI members should ensure that the Bangui Agreement fully and optimally incorporates the transition periods as well as TRIPS flexibilities available to LDCs and avoids TRIPS plus provisions.
- A review of the recently amended Bangui Agreement should be undertaken to examine the extent to which TRIPS flexibilities are incorporated and to identify TRIPS plus provisions.
- OAPI Secretariat should implement Article 46 of the recently amended Bangui Agreement by ensuring that pharmaceutical patents granted do not apply to LDCs in the OAPI region.
iii.For Developing Countries
- Provide technology transfer to LDCs in local pharmaceutical production
- Support LDCs negotiating to join the WTO and ensure that conditions of accession do not compromise transition periods, TRIPS flexibilities or include TRIPS-plus demands.
iv. For Developed Countries
- Fully support LDCs in use of the transition periods and refrain from setting any conditions for LDCs in WTO accession, transition period requests or FTA negotiations that impose TRIPS-plus measures, undermine their right to use TRIPS flexibilities or pressure the world’s poorest countries into early compliance with the TRIPS Agreement. 8
- Fulfill obligation under the TRIPS Agreement and provide incentives for genuine technology transfer.
v. For WIPO and Other Agencies (e.g. EPO, JPO, USPTO) providing Technical Assistance
- Undertake an independent review of technical assistance being provided by WIPO and other agencies to LDCs, to assess its suitability for LDCs from a development and public health perspective.
- WIPO and other agencies providing technical assistance to LDCs should ensure that LDCs fully and optimally utilize the transition periods and other TRIPS flexibilities.
- WIPO and other agencies should provide full information to the public on the type of information and legal advise being provided to LDCs with regard to IP implementation.
- Independent monitoring, evaluation and accountability mechanisms should be established with regard to technical assistance being provided to LDCs by WIPO and other agencies.
- LDCs should not accede to IP Conventions that facilitates protection of IP such as the Patent Cooperation Treaty, the Patent Law Treaty. Where LDCs are already Contracting Parties, LDCs should be exempted from the obligations of such instruments.
- The United Nations Technology Bank for LDCs should review its approach to IP44 and amend it to bring it in line with the achievement of right to health in LDCs. As stated by UNDP and UNAIDS: “…without the requirement of providing intellectual property protections, LDCs are free to follow the historic path of copying and adaptation to develop their technological capacities, at the same time strengthening their human, administrative, financial and other capacities…” 45. It is imperative that the Technology Bank promotes the utilization of transitions periods and full use of TRIPS flexibilities by LDCs.
These recommendations are aimed at remedying policy incoherence and maximizing policy space for LDCs to facilitate access to pharmaceutical products, which in turn will have a positive impact on public health and enable fulfillment of human rights.
Bibliography and References
1 More than 70% of the LDC population lives on less than $2 per day, according to the UN
2 The majority of the LDCs (34) are in the Sub-Saharan region.
3 Based on gross national income (GNI) per capita, the human asset index (HAI), economic vulnerability
index (EVI)
4 For example according to data available 38% of the Ugandan population, 68% of the Tanzanian
population, 63% of the Rwandan population, 81% of the Burundi population lives on less than $1.25.
Access to electricity in 2012, was just 34% in LDCs, compared to 85% in World. GNI per capita for LDCs
was US$928 as at 2014, compared to US$44289 for high-income countries and $10,857 for the World.
LDCs represent nearly 13 percent of world population, but only 1% of the global income. LDCs are at the
bottom of the human development index.
5 Out of the 20 member countries of the g7+ group of countries in post/conflict situations and fragile
states, 18 are LDCs.
6 For example it is reported that in Uganda only 22% of children (0-14) and 40% of adults living with HIV
are receiving ARVs while in Tanzania only 16% of children and 41% of adults living with HIV are
receiving ARVs6. Overall at the end of 2013, 63% of the 10.7m people living with HIV in LDCs did not have
access to ARV therapy.
7 According to a WHO Status Report of 2010, on non-communicable diseases, in the African Region, a
region with many LDCs, the prevalence of NCDs is rising rapidly and is projected to cause almost three quarters as many deaths as communicable, maternal, perinatal, and nutritional diseases by 2020, and to
exceed them as the most common causes of death by 2030. In the specific case of cancer, data from low income countries suggests that cancer incidence is expected to rise by 82% from 2008 to 2030, whereas
in high-income countries incidence is expected to rise at a much lower rate of 40%, in part due to
widespread access to vaccines and medicines.
8 For e.g. the 2008 WHO Global Strategy and Plan of Action on Public Health, Innovation and Intellectual
Property, the 2011 UN General Assembly Declaration on HIV and AIDS, the 2012 UN General Assembly
resolution on UHC, the 2013 WHO Global Action Plan for the Prevention and Control of NCDs 2013-2020.
9 The history of intellectual property and development does not support the proposition that strong IP
can be a driver of development. The history of today’s developed countries reveals that they went through
a period of using both legitimate and illegitimate means to acquire foreign technologies to support
nascent industries in order to become competitive. In the 16th and 17th century pre-IP era, Britain
borrowed manufacturing methods, skilled artisans, and machinery from Venice and so-called Low
Countries to develop its wool- and silk-based industries. Thereafter, France, Russia, Sweden, Norway,
Denmark, the Netherlands, and Belgium engaged in industrial espionage, often state supported, to obtain
advanced technologies from England. Most advanced countries were still routinely violating the IPRs of
other countries’ citizen well into the 20th century. According to Ha-Joon Chang: “....when they were
backward themselves in terms of knowledge, all of today’s rich countries blithely violated other people’s
patents, trademarks and copyrights. The Swiss “borrowed” German chemical inventions, while the
Germans “borrowed” English trademarks” and the Americans “borrowed” British copyrighted materials –
all without paying what would today be considered “just” compensation « . The experience of many
Asian countries that build technological capacity is also similar. See Ha-Joon Chang ‘Intellectual Property
Rights and Economic Development – Historical Lessons and Emerging Issues’ Third World Network
Intellectual Property Rights Series No 3 2001, available at http://www.twn.my/title2/IPR/pdf/ipr03.pdf
and Ha-Joon Chang ‘Kicking Away the Ladder: the “Real” History of Free Trade’ Foreign Policy in Focus
Special Report (2003), available at
http://www.fpif.org/reports/kicking_away_the_ladder_the_real_history_of_free_trade. See also Chapter 1 of
UK Commission Report on Intellectual Property at
http://www.iprcommission.org/papers/pdfs/final_report/Ch1final.pdf. See Richard Gerster, “Patents
and Development: Lessons learnt from the economic history of Switzerland”,
http://www.twn.my/title2/IPR/pdf/ipr04.pdf
10 WIPO’s technical assistance is heavily criticized as being against public interest and development needs
as well as inadequate and inappropriate on flexibilities. See WIPO Doc IIM/1/4 “Proposal to Establish A
Development Agenda for WIPO” at
https://www.google.co.uk/url?sa=t&rct=j&q=&esrc=s&source=web&cd=3&ved=0ahUKEwiE1K7L5JfLAh
XIORQKHTVCAQ4QFggkMAI&url=http%3A%2F%2Fwww.wipo.int%2Fedocs%2Fmdocs%2Fmdocs%2Fen%2Fiim_1%2Fiim_1_4.doc&usg=AFQjCNHdVUFQMzh9o8DKOMRiW8IleVMY0Q&sig2=vR2P4hoinLe_BAyFmhkvvQ; See also An External Review of WIPO Technical Assistance in the Area of Cooperation for Development (CDIP/8/INF/1) at http://www.wipo.int/meetings/en/doc_details.jsp?doc_id=254006. This independent review is critical of WIPO’s technical assistance and has even found examples of TRIPS plus advice being given to developing countries and LDCs. Further see also Expert Review Calls For Technical Assistance Reforms, Further Investigation by Sangeeta Shashikant (15 Nov 11); WIPO: Technical assistance criticized for shortcomings by Sangeeta Shashikant (14 Nov 11)
11According to Prof. Peter Drahos, “[o]ver the years the steady drip of technical assistance leads to the
formation of technocratic trust in the EPO’s systems. A strong belief forms that the EPO’s systems produce
quality results and that belief in turn forms the basis of decision-making by patent examiners in underresourced developing country patent offices. Technocratic trust thus fosters a circle of decision-making in which the EPO trains developing country examiners to make decisions in their own countries that
predominantly benefit foreign companies, including European companies”. Peter Drahos, “Trust Me”:
Patent Offices in Developing Countries, Working Paper, Centre for Governance of Knowledge and
Development <http://www.anu.edu.au/fellows/pdrahos/pdfs/2007Drahostrustmessrn.pdf; See also NGO letter to WIPO on organizing “Africa IP Summit: Lacking a Development Dimension” with the US Department of Commerce and a number of large US corporations (Microsoft, Eli Lilly. Pfizer, etc).
http://www.ghwatch.org/sites/www.ghwatch.org/files/AfricaIPSummit2012_0207.pdf
12 Shashikant Sangeeta (2014) “The African Regional Intellectual Property Organization (ARIPO)”:
Implications for Access to Medicines” South Centre Research Paper 56 available at
http://www.southcentre.int/wp-content/uploads/2014/11/RP56_The-ARIPO-Protocol-onPatents_ENl.pdf;
Drug patents in French-speaking Africa” available at
http://www.eldis.org/go/home&id=29848&type=Document#.VtGGhikpSWA; “Regional Issue Brief:
Intellectual Property & Access to Medicines” Intellectual property rights and access to medicines, Global
Commission on HIV and Law.
13 Preamble of the TRIPS Agreement states: “Recognizing also the special needs of the least-developed
country Members in respect of maximum flexibility in the domestic implementation of laws and
regulations in order to enable them to create a sound and viable technological base.”
14 See WTO doc. IP/C/64 at https://www.wto.org/english/news_e/news13_e/trip_11jun13_e.htm
15 See WTO doc IP/C/73.
16 See WTO doc WT/L/971
17 Article 66.1 of the TRIPS Agreement: “The Council for TRIPS shall, upon duly motivated request by a
least-developed country Member, accord extensions of this period”.
18 See Communication From Bangladesh On Behalf Of The LDC Group, WTO Doc. IP/C/W/605 (23
February 2015) and Request for an Extension of the Transitional Period, Under Article 66.1 of the TRIPS
Agreement from Haiti, WTO Doc. IP/C/W/583 (5 November 2012).
19 US stands in the way of LDCs' pharmaceutical transition period; "Unconscionable and indefensible" -
U.S. 10-year offer to LDCs for pharmaceutical patent waiver; US and EU demand TRIPS plus concession
from poorest countries (30 Apr 13) ; Academics Worldwide Support LDCs' Request for TRIPS Extension
(29 Apr 13) ; LDC Watch concerned with developed countries' position on LDC extension (27 Apr 13)
20 For e.g. Afghanistan, Bangladesh, Democratic Republic of the Congo, Madagascar, Myanmar and Nepal.
21 Allows patent applicants to file patent applications simultaneously in multiple jurisdictions
22 History of IP is full of examples about the ways in which developed countries adapted the IP rules to
their changing needs and how the levels of protection increased as their industrial and technological
capacities improved over time. Japan: benefitted from IP generated in other developed countries. Its
patent protection was designed with an ultimate objective of contributing to the industrial development.
For e.g. until 1975 it excluded food, beverage, pharmaceutical products and chemical compounds from the
scope of patent protection. This weak patent protection is how Japan obtained technology and facilitated
its absorption. There were complaints of discrimination by the Japan Patent Office in that foreign
applicants had to wait longer to obtain patents compared to domestic applicants. It only increased patent
protection on receiving pressure from the US. In any case by then Japanese enterprises that developed
significantly and were developing their own innovations. Taiwan: employed weak policy to facilitate local
absorption of foreign knowledge through reverse engineering. Its government openly encouraged
counterfeiting as a strategy to develop local industries. Only under pressure from the US beginning 1983 it instituted stronger IP law. Case of Switzerland: For example in the 19th century, Switzerland’s
chemicals and textiles industries were strongly opposed to the introduction of patents as it would restrict
their copying of processes abroad. Then Switzerland was a poor country without many natural resources,
whose economy was largely reliant on farming. In 1859 a small company based in Basel “borrowed” the
aniline dying process which had been developed and patented in Britain two years before. The company,
later called Ciba, soon became a massive industrial enterprise, swiftly outstripping competing firms in
Britain. In 1995, Ciba merged with another Swiss firm, Sandoz, to form the conglomerate
Novartis…..currently a huge multinational pharmaceutical company. Case of United States: US was a
notorious pirate particularly of English work. For almost 100 years the US refused to grant copyright
protection to foreign authors on the grounds it was important to meet the nations needs for knowledge
and enlightenment and to reduce deficit in international royalty payments. As a result American publishes
and producers freely pirated foreign literature.
23 Zanzibar (part of Tanzania) excludes both pharmaceutical products and processes from patent
protection.
24 If countries fail to object to the grant of pharmaceutical patents as required by the Harare Protocol (i.e.
6 months following notification of a grant by the ARIPO Secretariat).
25 The African Intellectual Property Organization (ARIPO): Implications for Access to Medicines, Sangeeta
Shashikant, Research Paper 56, South Centre. See http://www.southcentre.int/wpcontent/uploads/2014/11/RP56_The-ARIPO-Protocol-on-Patents_ENl.pdf
26 It is recommended by the UN, agencies, the UK’s Commission on Intellectual Property Rights and the
WHO’s Commission on Intellectual Property, Innovation and Public Health (CIPIH). See also Correa, Carlos M. 2007a. Guidelines for the examination of pharmaceutical patents: developing a public health
perspective. A working paper. Geneva: International Centre for Trade and Sustainable Development,
United Nations Conference on Trade and Development and World Health Organization. Available at
http://ictsd.org/downloads/2008/06/correa_patentability20guidelines.pdf
27 The Zanzibar patent law prohibits patents on new uses and new forms of known substances. Samoa's
patent law includes a provision similar to Section 3(d) of India's patent law while Burundi and Rwanda
exclude patents on new uses of known substances.
28 Shashikant, Sangeeta. 2014. The African Regional Intellectual Property Organization (Aripo) Protocol
On Patents: Implications For Access To Medicines. Research Paper No. 56. South Centre: Geneva. Available at http://www.southcentre.int/wp-content/uploads/2014/11/RP56_The-ARIPO-Protocol-onPatents_ENl.pdf
29 Shashikant, Sangeeta. 2014. The African Regional Intellectual Property Organization (Aripo) Protocol
On Patents: Implications For Access To Medicines. Research Paper No. 56. South Centre: Geneva. Available
at http://www.southcentre.int/wp-content/uploads/2014/11/RP56_The-ARIPO-Protocol-onPatents_ENl.pdf
30 For example, Cambodia, Uganda, Yemen, Burundi and Sierra Leone recognise the international
exhaustion rule for parallel importation. Others such as Bhutan, Madagascar, and Lesotho only recognise
national exhaustion limiting their ability to parallel import cheaper pharmaceutical products from other
countries.
31 ASEAN Working Group on Intellectual Property Cooperation (AWGIPC). Undated. ASEAN Intellectual
Property Rights Action Plan 2011-2015. Available at
https://www.aseanip.org/Portals/0/PDF/ASEAN%20IPR%20Action%20Plan%202011-2015.pdf
32 As noted by the Global Commission on HIV and Law, "the recent proliferation of anti-counterfeiting
legislation in East Africa, promoted by multinational pharmaceutical companies, reflects the increasing
conflation of generic with counterfeit drugs— substandard formulations that endanger people who take
them—and the myth that generics are inferior to originator brands." See also Kenya’s High Court Strikes
down Anti-Counterfeit Act, https://www.opensocietyfoundations.org/press-releases/kenya-s-high-courtstrikes-down-anti-counterfeit-act
33 While some like Yemen and Mozambique provide for fines for infringement, those like Cambodia,
Angola, Cape Verde, Madagascar, Lesotho, South Sudan, Rwanda and Sierra Leone also provide for
imprisonment.
34 Two (Samoa and Cape Verde) have since graduated from the LDC status and the entry of Afghanistan
and Liberia that was approved in December 2015 will be finalised by mid-2016.
35 For instance, the two most recent accessions, based on the limited information released by the WTO
seem to imply that Afghanistan has a transition period to comply with TRIPS only till 2019 and a no-roll
back commitment while Liberia's accession commitments on TRIPS appear to be simply that it will
comply with TRIPS. Pressure on LDCs regarding data exclusivity featured explicitly in the working party
reports of Cape Verde, Vanuatu and Yemen.
36 See WTO Doc. WT/COMTD/LDC/21
37 “We reaffirm the commitment of developed-country members to provide incentives to their
enterprises and institutions to promote and encourage technology transfer to least-developed country
members pursuant to Article 66.2.” WTO Ministerial Conference. 14 November 2001. Declaration on the
TRIPS Agreement and Public Health. Para 7
38A review of the reports filed by developed countries with the WTO between 1999-2010 found that " of
384 unique programmes or policies reviewed, 33% were targeted specifically towards LDC WTO
Members...[o]f the 128 programmes that specifically targeted LDC WTO Members, about one-third (42
programmes) qualified as technology transfer according to the definition we adopted. If we consider the
full set of 384 programmes listed by the reporting developed countries, only 11% met the criteria of
targeting an LDC WTO Member with a programme or policy that encourages technology transfer."; See
Suerie Moon 2011 http://www.ictsd.org/downloads/2011/05/technology-transfer-to-the-ldcs.pdf
39 “Local production of pharmaceuticals in Africa and access to essential medicines: 'urban bias’ in access
to imported medicines in Tanzania and its policy implications”, Mujinja, 2014,
http://globalizationandhealth.biomedcentral.com/articles/10.1186/1744-8603-10-12
40African Union. (2012b). “Pharmaceutical Manufacturing Plan for Africa Business Plan”, prepared as part
of the AUC-UNIDO partnership, Addis Ababa, 2012; EAC Regional Pharmaceutical Manufacturing Plan of
Action (2012- 2016); the South African Development Community Pharmaceutical Business Plan (2007-
2013); the Economic Community of West African States (ECOWAS) Regional Pharmaceutical Plan,
UNAIDS 1 May 2014.
41 African Union. (2012b). “Pharmaceutical Manufacturing Plan for Africa Business Plan”, prepared as part
of the AUC-UNIDO partnership, Addis Ababa, 2012. Available from
http://apps.who.int/medicinedocs/documents/s20186en/s20186en.pdf
42 See Moon Surie. December 2008. Does TRIPS Art. 66.2 Encourage Technology Transfer to LDCs? An
Analysis of Country Submissions to the TRIPS Council (1999-2007). UNCTAD - ICTSD Project on IPRs and
Sustainable Development. Policy Brief Number 2. Available at
http://www.iprsonline.org/New%202009/Policy%20Briefs/policy-brief-2.pdf. See also Correa, Carlos
M. 2007. Intellectual Property in LDCs: Strategies for Enhancing Technology Transfer and Dissemination.
The Least Developed Countries Report 2007. Background Paper. UNCTAD. Available at
http://unctad.org/sections/ldc_dir/docs/ldcr2007_Correa_en.pdf
43 Joint Resolution of the Ministry of Industry, Ministry of Health and Instituto Nacional de la Propiedad
Industrial 118/2012, 546/2012 y 107/2012. See http://www.moellerip.com/non-patentable-subjectmatter-
according-to-the-new-guidelines-of-the-argentine-pto-2/
44 The proposal for Technology Transfer Bank is based on flawed assumptions that IP laws and
enforcement in LDCs must underlie this technology transfer and the work outlined for the "IP Bank" in
this proposal. See http://unohrlls.org/custom-content/uploads/2015/10/Feasibility-Study-ofTechnology-Bank.pdf
45 UNDP (United Nations Development Programme) and Joint United Nations Programme on HIV/AIDS
(UNAIDS). 2013. TRIPS transition period extensions for least-developed countries. Issue Brief. Geneva
and New York: UNAIDS and UNDP. Available at
http://www.unaids.org
1. Abbott, Frederick M. May 2013. Technical Note: The LDC TRIPS Transition Extension and the Question of Rollback. Policy Brief No. 15, (2013) Geneva, Switzerland: International Centre for Trade and Sustainable Development (ICTSD). Available at http://ssrn.com/abstract=2273409..
2. Baker, B. Debunking IP-for-Development: Africa Needs IP Space Not IP Shackles in INTERNATIONAL ECONOMIC LAW AND AFRICAN DEVELOPMENT, 82-110 (Laurence Boulle, Emmanuel Laryea & Franziska Sucker eds.2014)
3. Birkbeck, Carolyn Deere and Roca, Santiago. 2011. An External Review of WIPO Technical Assistance in the Area of Cooperation for Development. CDIP/8/INF/1. WIPO. Available at http://www.wipo.int/meetings/en/details.jsp?meeting_id=22206
4. Center for Health, Human Rights and Development (CEHURD) and UNDP. Undated. Promoting Local Pharmaceutical Production in Uganda: Challenges facing local pharmaceutical firms. CEHURD: Kampala
5. Chea, Samnang and Sok, Hach. 2013. Cambodia's Membership in the WTO and the Implications for Public Health. Yale Journal of Health Policy, Law, and Ethics. Volume 4. Issue 2. Article 9. Available at: http://digitalcommons.law.yale.edu/yjhple/vol4/iss2/9
6. Commission on Intellectual Property Rights. September 2002. Integrating Intellectual Property Rights and Development Policy. London. Available at http://www.iprcommission.org/papers/text/final_report/reporthtmfinal.htm
7. Committee on Economic, Social and Cultural Rights. 11 May 2000. General Comment No. 14 (2000): The right to the highest attainable standard of health (art. 12 of the Covenant). E/C.12/2000/4.
8. Correa, Carlos M. 2000. Integrating public health concerns into patent legislation in developing countries. Geneva: South Centre. Available at http://apps.who.int/medicinedocs/pdf/h2963e/h2963e.pdf
9. Correa, Carlos M. 2007b. Intellectual Property in LDCs: Strategies for Enhancing Technology Transfer and Dissemination. The Least Developed Countries Report 2007. Background Paper. UNCTAD. Available at http://unctad.org/sections/ldc_dir/docs/ldcr2007_Correa_en.pdf
10. Correa, Carlos M. 2012. Beyond ‘patent quality’: basic concepts of the patent system need to be reviewed. South Bulletin.
11. Correa, Carlos M. 2014. Tackling The Proliferation Of Patents: How To Avoid Undue Limitations To Competition And The Public Domain. Research Paper No. 52. South Centre: Geneva. Available at http://www.southcentre.int/wp-content/uploads/2014/09/RP52_Tackling-the-Proliferation-ofPatents-rev_EN.pdf
12. Drug seizures in Frankfurt spark fears of EU-wide pattern. IP-Watch. 5 June 2009 Available at http://www. ip-watch.org/2009/06/05/drug-seizures-in-frankfurt-spark-fears-of-eu-wide-pattern
13. EAC. February 2013. East African Community Regional Intellectual Property Policy on the Utilisation of Public Health-Related WTO-TRIPS Flexibilities and the Approximation of National Intellectual Property Legislation 14
14. European Commission. 10 September 2015. European Commission supports better access to medicines in poorest countries. Press Release. Brussels
15. Global Commission on HIV and the Law. 2012. Risks, Rights, and Health. New York, NY: United Nations Development Programme. Available at http://www.hivlawcommission.org/resources/report/FinalReport- Risks,Rights&Health-EN.pdf
16. Grover A. 31 March 2009. Report of the Special Rapporteur on the right of everyone to the enjoyment of the highest attainable standard of physical and mental health.A/HRC/11/12. Available at http://www.ifhhro.org/images/stories/ifhhro/documents_UN_special_rapporteur/3_4_1_en.pdf
17. Kapczynski A, Park C, Sampat B. 2012. Polymorphs and prodrugs and salts (Oh My!): an empirical analysis of “secondary” pharmaceutical patents. PLOS One 2012;7(12). Available at http://www.plosone.org/ article/info%3Adoi%2F10.1371%2Fjournal.pone.0049470
18. Khan, Z., Intellectual Property and Economic Development: Lessons from American and European History. See http://www.iprcommission.org/papers/pdfs/study_papers/sp1a_khan_study.pdf
19. Kumar N., Intellectual Property Rights, Technology and Economic Development: Experiences of Asian Countries. See http://www.iprcommission.org/papers/pdfs/study_papers/sp1b_kumar_study.pdf
20. Mathews, Duncan and Munoz-Tellez, Viviana. 2006. Bilateral Technical Assistance and TRIPS: The United States, Japan and the European Communities in Comparative Perspective. The Journal of World Intellectual Property (2006) Vol. 9, no. 6, pp. 629–653.
21. Medecins Sans Frontieres (MSF). 2003. Doha Derailed: A Progress Report on TRIPS and Access to Medicines. Available at https://www.msfaccess.org/sites/default/files/MSF_assets/Access/Docs/ACCESS_report_DohaDer ailed_ENG_2003.pdf
22. Musungu SF, Oh C. The use of TRIPS flexibilities by developing countries: can they promote access to medicines? Commission on Intellectual Property Rights, Innovation and Public Health, Study 4C. Available at http://www.who.int/intellectualproperty/studies/TRIPSFLEXI.pdf.
23. Patricia AseroOchieng and Ors. v. Attorney General and Anr., Petition 409 of 2009, High Court of Kenya, Date of Decision: 20 April 2012
24. Seuba X, Rovira J, Bloemen S. Welfare Implications of Intellectual Property Enforcement Measures. PIJIP Research Paper no. 5. Washington DC: American University Washington College of Law, 2010. Available at http://digitalcommons.wcl.american.edu/research/5/.
25. Shashikant, Sangeeta. 2014. The African Regional Intellectual Property Organization (Aripo) Protocol On Patents: Implications For Access To Medicines. Research Paper No. 56. South Centre: Geneva. Available at http://www.southcentre.int/wp-content/uploads/2014/11/RP56_The-ARIPOProtocol-on-Patents_ENl.pdf
26. Sixty-first World Health Assembly. May 2008. Global strategy and plan of action on public health, innovation and intellectual property. Resolution WHA61.21. Available at http://apps.who.int/ gb/ebwha/pdf_files/A61/A61_R21-en.pdf
27. Third World Network. 16 June 2009. Concerns voiced at TRIPS Council over seizure of drugs. Third World Network Info Service on Health Issues.Available at http://www.twnside.org.sg/title2/health.info/2009/ twnhealthinfo20090602.htm
28. Thorpe P. Study on the Implementation of the TRIPS Agreement by Developing Countries. Commission on Intellectual Property Rights, Study Paper 7. Available at http://www.iprcommission.org/ papers/pdfs/study_papers/sp7_thorpe_study.pdf.
29. UNAIDS, UNDP and WHO. 2011. Using the TRIPS Flexibilities to Improve Access to Treatment. Policy Brief. Available at http://content.undp.org/go/cms-service/stream/asset/?asset_id=3259398.
30. UNAIDS. 1 May 2014. ECOWAS and partners to boost the local production of quality medicines. Press Release 15
31. UNAIDS. 2011. Implementation of TRIPS and Access to Medicines for HIV after January 2016: Strategies and Options for Least Developed Countries. Technical Brief. Available at http://www.unaids.org/sites/default/files/media_asset/JC2258_techbrief_TRIPS-accessmedicines-LDC_en_0.pdf
32. UNCTAD. 2011. Local Production of Pharmaceuticals and Related Technology Transfer in Developing Countries: A series of case studies. UNCTAD/DIAE/PCB/2011/7
33. UNDP (United Nations Development Programme) and Joint United Nations Programme on HIV/AIDS (UNAIDS). 2013. TRIPS transition period extensions for least-developed countries. Issue Brief. Geneva and New York: UNAIDS and UNDP. Available at http://www.unaids.org/sites/default/files/media_asset/JC2474_TRIPS-transition-periodextensions_en_0.pdf
34. UNDP. 2010. Good practice guide: improving access to treatment by utilizing public health flexibilities in the WTO TRIPS Agreement. New York, NY: UNDP. Available at http:// apps.who.int/medicinedocs/documents/s17762en/s17762en.pdf
35. UNDP. 2012. Anti-counterfeit laws and public health. Discussion Paper. Available at http://www.undp.org/content/dam/undp/library/hivaids/English/UNDP%20Discussion%20Pape r%20-%20(revised).pdf
36. United Nations Department of Economic and Social Affairs (UN DESA). Undated. LDC Information: Graduation and Transition Process. Available at http://www.un.org/en/development/desa/policy/cdp/ldc/ldc_graduated.shtml
37. United Nations General Assembly. 10 June 2011. Political Declaration on HIV and AIDS: Intensifying Our Efforts to Eliminate HIV and AIDS. A/RES/65/277. Available at http://www.unaids.org/sites/default/files/sub_landing/files/20110610_UN_A-RES-65-277_en.pdf
38. United Nations General Assembly. 25 September 2015. Transforming our world: the 2030 Agenda for Sustainable Development. A/RES/70/1
39. UNOHRLLS. 2014. List of Least Developed Countries in 2014. Available at http://www.un.org/en/development/desa/policy/cdp/ldc/ldc_list.pdf
40. UN-OHRLLS. 2015. Criteria for Identification and Graduation of LDCs. Available at http://unohrlls.org/about-ldcs/criteria-for-ldcs/
41. WHO SEARO and WHO PAHO. 2006. Data Exclusivity And Other “TRIPS-Plus” Measures. Briefing Note: Access to Medicines. Available at http://www.searo.who.int/entity/intellectual_property/data-exclusively-and-others-measuresbriefing-note-on-access-to-medicines-who-2006.pdf
ACKNOWLEDGMENTS: This submission relies extensively on a review of LDC patent laws done by Kajal Bhardwaj as part of a fellowship at the Indian Institute of Advanced Studies and on an extensive study of ARIPO by Sangeeta Shashikant for the South Centre. Professor Brook K. Baker contributed to its conceptualization and drafting