Submission: UNITAID
Prepared by: Philippe Duneton, Karin Timmermans, Carmen Pérez Casas
Country: Switzerland
Abstract
The key points of this submission are:
• Timely access to optimized medicines can be hampered by patents and other intellectual property rights (IPR), as well as by barriers that are not related to IPR. These other barriers equally need to be addressed in order to effectively ensure rapid access to medicines for people in low-and middle-income countries.
• In order to effectively contribute to and accelerate the global response, it is important to have a holistic view of all aspects that affect access to medicines and to address all challenges along the value chain.
Submission
UNITAID SUBMISSION TO THE UNITED NATIONS SECRETARY-GENERAL’S HIGH-LEVEL PANEL ON ACCESS TO MEDICINES
ACCELERATING ACCESS TO INNOVATION: LESSONS LEARNED BY UNITAID
About UNITAID
UNITAID is engaged in finding new ways to prevent, treat and diagnose HIV/AIDS, tuberculosis (TB) and malaria more quickly, more cheaply and more effectively. It takes game-changing ideas and helps to turn them into practical solutions that can help accelerate the end of the three diseases. Established in 2006 by Brazil, Chile, France, Norway and the United Kingdom to provide an innovative approach to global health, UNITAID plays an important part in the global effort to defeat HIV/AIDS, tuberculosis and malaria, by facilitating and speeding up the availability of improved health tools, including medicines and diagnostics. UNITAID identifies health solutions that show promise and invests in them to enable their scale-up, working closely with partner organizations to ensure these solutions reach those most in need. By helping to fast-track access and reduce costs of new more effective medicines, technologies and systems UNITAID can maximize the impact of every dollar spent to overcome these lethal diseases.
To date, UNITAID’s work has focused on HIV, TB and malaria. Currently, UNITAID is developing its next five-year strategy (2017-2021); alignment with the recently adopted Sustainable Development Goals is one of the guiding principles in this process.
Key points
In response to the High Level Panel’s call for contributions that address the policy incoherence in relation to the rights of inventors, international human rights law, trade rules, and public health objectives including increased access to medicines and other health technologies, UNITAID has submitted a paper providing data on the actual and projected impact of strategies for overcoming patent barriers, where they exist. It is based on UNITAID’s experience and lessons learnt in that field to date. In addition, UNITAID would furthermore like submit the following.
The key points of this submission are:
• Timely access to optimized medicines can be hampered by patents and other intellectual property rights (IPR), as well as by barriers that are not related to IPR. These other barriers equally need to be addressed in order to effectively ensure rapid access to medicines for people in low-and middle-income countries.
• In order to effectively contribute to and accelerate the global response, it is important to have a holistic view of all aspects that affect access to medicines and to address all challenges along the value chain.
If the different elements that need to be in place to ensure countries can benefit from game-changing tools are not addressed simultaneously, people in need will only have access to key treatment, preventives or diagnostics tools with a large time lag. For example, although newer promising antiretroviral medicines, such as dolutegravir, have been included in voluntarily licences with the Medicines Patent Pool and generic products could be manufactured and sold in many countries in need, this advantageous treatment is not yet available because of lack of evidence on its use and lack of adequate combinations.
Accelerating the response
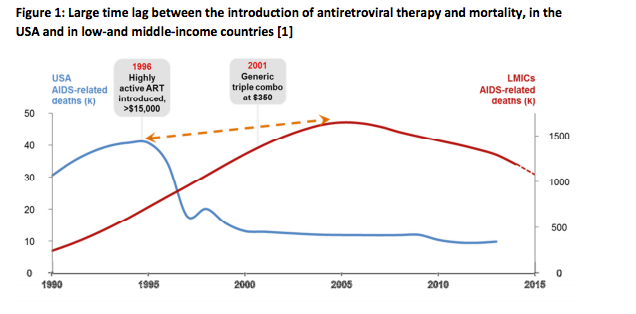
The history of HIV illustrates the importance of innovation and access. Due to the prohibitive cost of emerging medicines, AIDS-related mortality continued to soar in low-and middle-income countries for many years, while AIDS-related mortality declined in the USA, following scale up of highly-active antiretroviral therapy (HAART) in the USA (where the introduction of HAART triggered a 75% drop in AIDS-related mortality in a period of three years). It wasn’t until 2001, when cheaper generic fixed-dose combinations became available in low-and middle-income countries, that mortality started to decline (see Figure 1). [1].
Figure 1: Large time lag between the introduction of antiretroviral therapy and mortality, in the USA and in low-and middle-income countries [1]
The ambitious goals that the international community has set itself (ending the epidemics of AIDS, tuberculosis and malaria by 2030) will require a paradigm shift in the global response to these diseases in the coming years. It is imperative to move away from a business-as-usual approach to an accelerated response, underpinned by the accelerated use of cutting-edge technologies and innovative approaches in the countries where the majority of the disease burden is occurring.
Challenges along the value chain
The uptake of optimized medicines to treat HIV, TB or malaria in low- and middle income countries often is slow. This can be due to a variety of reasons, ranging from patient factors (e.g. a lack of awareness and demand), to health system issues, financing or factors affecting the production and supply of medicines. UNITAID focuses on market factors that affect access; notably:
• Patents can hamper the development of generic medicines (including fixed-dose combinations). Thus, they can hamper competition. Strategies to overcome patent barriers exist; these notably include pooled voluntary licensing through the Medicines Patent Pool and the use of TRIPS flexibilities. Both these approaches can have significant impact, as shown in UNITAID’s paper on the actual and projected impact of strategies. Pooling voluntary licenses also has a large benefit in facilitating and accelerating the development of fixed-dose combinations of WHO recommended regimens, in particular as the Medicines Patent Pool now has licenses to most WHO recommended antiretrovirals.
• Lack of competition. In the absence of generic competition, prices are and will be unaffordable. The lack of competition can be due to patents, but other factors can equally play a role, for example registration hurdles. Sales volumes – or the lack thereof – can also have a significant impact on volumes, and thus on prices and affordability.
• Registration-related barriers. Regulatory approval, or authorization by relevant authorities, is a precondition for the use of any medicine1. However, the approval and assessment process at country level can be time consuming. For instance, in several countries, TDF/FTC is approved as treatment for HIV, but not for use as prophylaxis; this can constrain its use for pre-exposure prophylaxis (PrEP). See also Box 1.
Where it exists, linkage between patent status and registration may result in additional delays for the registration of generic products. Data exclusivity is another hurdle2. Information about data exclusivity is not always easily available in low- and middle income countries that provide this type of exclusive rights. Where granted, there may be no mechanisms or procedures for exemptions to data exclusivity, even if this were to be in the interest of public health.
• Lack of data relevant for low-and middle-income countries. There may be a lack of clinical data pertaining to the use of a medicine in a specific target group, specifically in the context of low- and middle income countries where products will be used by millions. Furthermore, there data may be lacking for a potentially advantageous combination therapy, especially when combining medicines developed by different originator companies. An example is dolutegravir, which has been recognized as a “potential game-changer” for treatment of HIV [2]. Dolutegravir has been approved by the European Medicines Agency and the United States Food and Drug Administration and is recommended by WHO as a component of first-line regimens [3]. However, to date, studies and trials with dolutegravir have not included significant numbers of, for example, pregnant women or people co-infected with HIV and tuberculosis (who represent only a small percentage of the population on antiretroviral treatment in high-income markets countries, but make up a more significant portion of people on treatment in low-and middle-income countries) [4]. The lack of information, including data on the safety and pharmacology, may hamper the uptake and registration of innovative medicines such as dolutegravir in low-and middle-income countries.
• Lack of adapted formulations. Certain formulations, such as paediatric medicines3 or fixed-dose combinations may not exist. This may be due to patent barriers, but there can also be other constraints, such as technological challenges; for example, it can be very difficult to mask the bitter taste of some medicines in formulations for children and incentives to address such technological challenges may lack. Fixed dose combinations are crucial for simplifying treatment, improving adherence and scaling up. See also Box 1.
• Meeting quality standards. It is imperative that medicines are of assured quality [5]. Donor agencies, such as the Global Fund and PEPFAR, have procurement policies requiring that health products meet strict quality standards to become eligible for procurement with their funds.
• Catalytic procurements and forecasting of needs. Forecasting the future requirements of medicines and other health products in a timely and reliable manner is key. It helps to generate interest and incentivize production by generic manufacturers; thus, it can help promote competition, which in turn contributes to making products affordable.
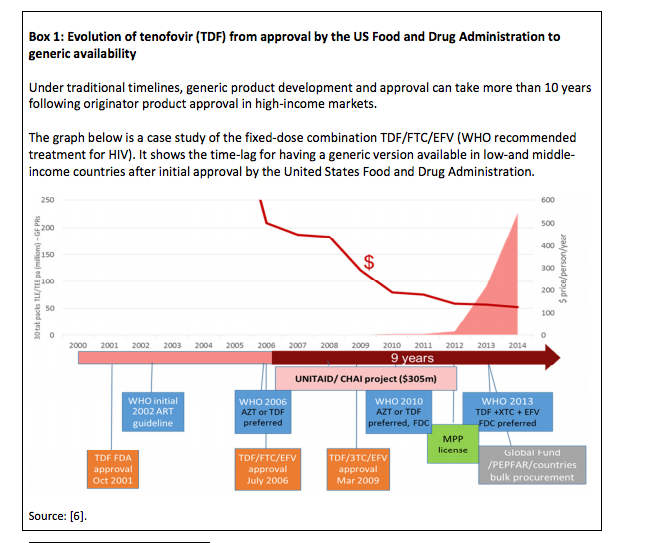
Under traditional timelines, generic product development and approval can take more than 10 years following originator product approval in high-income markets.
The graph below is a case study of the fixed-dose combination TDF/FTC/EFV (WHO recommended treatment for HIV). It shows the time-lag for having a generic version available in low-and middle-income countries after initial approval by the United States Food and Drug Administration. Source: [6].
Considering current as well as future challenges
Through the projects it funds, UNITAID aims to solve current problems, but also attempts to take into consideration the challenges ahead, in order to address them early on and accelerate access to innovative products in low-and middle-income countries. The following section provides some examples of this approach.
WHO prequalification
The prequalification programme by WHO4 was designed to provide an independent verification of the quality, safety and efficacy of medicines procured by United Nations Agencies and of donor-funded medicines. National regulatory authorities may fast-track the registration of medicines that have been prequalified by WHO. Nevertheless, WHO prequalification, like any initiative to adequately verify the quality, safety and efficacy of a medicine, does take a certain time. Recognizing that in certain instances there is an urgent need to fast-track access to a particular medicine or health product, the World Health Organization, the Global Fund and UNITAID jointly promote a process of temporary approval, based on expert advice, that can allow the purchase of medicines that are not yet prequalified by WHO; this process is used when there are no or not enough sources of supply to ensure adequate access [7].
Leveraging licensing conditions
Voluntary licenses concluded by the Medicines Patent Pool (MPP)5 have an impact (see UNITAID submission “Overcoming patent barriers: options and impact”). They are forward looking in that they not only seek to address patent barriers, but they also help address quality and regulatory challenges:
• MPP licences include a provision that the patent-holder agrees to waive any applicable data-exclusivity rights in the countries included in the license, in order to facilitate rapid registration of generics in countries where data exclusivity might prevent this.
• The MPP requires companies that sign a sub-license to obtain WHO prequalification or tentative approval by the United States Food and Drug Administration for their products. This, too, will facilitate/accelerate their national registration in many countries, and furthermore ensures that the products are eligible for procurement by funders such as the Global Fund and PEPFAR.
• MPP licenses also provide transparency on patent status.
Obtaining data to accelerate uptake. UNITAID is currently considering funding projects that address the data gaps regarding dolutegravir (described above). These projects are also expected to contribute to evidence on dolutegravir’s cost-effectiveness under actual conditions of use. Importantly, these data may enable WHO to include dolutegravir in its guidelines, without exemption or restriction for certain patient groups. This, in turn, would facilitate their inclusion in national guidelines and accelerate their uptake. In order to increase the likelihood of this happening, UNITAID is collaborating closely with WHO, to ensure that the data resulting from these potential projects will meet WHO’s standards and requirements. By unlocking the market, these projects would contribute to decreasing prices. In addition, and very importantly in a simultaneous manner, while the necessary studies take place, interventions to prepare the market (supporting the development and price reduction of future combinations) take place.
Similarly, UNITAID is funding a project by Partners in Health, Médecins Sans Frontières and Interactive Research & Development that seeks to obtain evidence on the most appropriate and effective use of two new medicines for TB (bedaquiline and delamanid). These two new TB medicines are the first drugs approved for multi-drug resistant TB in more than forty years, but there is little data on how they can best be used in combination with other TB medicines or with each other.
Considering access during development. Ritonavir-boosted lopinavir (LPV/r) is a medicine recommended by WHO for treatment of HIV-infected infants and young children. In 2008, when WHO first recommended this medicine as the preferred first-line therapy for infants and young children, the only paediatric formulation available was a liquid with a high alcohol content that tastes terrible and requires refrigeration. Still, as of today, the guidelines cannot be adhered to, due to formulation issues. UNITAID supports the development for more suitable paediatric formulations of this medicine. Through funding the development of this and other new or adapted products6, UNITAID has learned that it is important to already consider access issues during the development stage of the product. In general it seems easier to “impose” conditions that support access (such as affordable pricing or a license that enables a third party to manufacture the product) early on in the development process.
A holistic response
In UNITAID’s experience, innovation is key to the response and to achieve global goals in HIV, TB and malaria. For UNITAID, innovation can take a variety of forms, which include, but are not limited to, the uptake of game-changing products. UNITAID seeks to accelerate innovative solutions that enable a more efficient and faster response, that provide value-for-money and that help countries, and partners supporting them, to do more with less.
In this context, addressing intellectual property issues is crucial, but it is not enough; rather it is necessary to have a holistic view of all the issues along the value chain. It is by working on all aspects of the value chain that the one can be most effective and maximize impact on the ground.
UNITAID has played a key role in promoting the use of first- and second line treatments currently recommended by WHO, such as TDF/FTC/EFV, in low-and middle-income countries, but it has taken 9 to 12 years (see Box 1). In order to achieve the ambitious Sustainable Development Goals, the uptake of new first- and second line treatments must be accelerated. UNITAID is working with countries and partners such as WHO, the Global Fund, the Medicines Patent Pool and others to reduce the time lag for the uptake of new medicines to less than three years.
Bibliography, References, and Notes
1. Strategic Narrative for HIV. Geneva: UNITAID (forthcoming)
2. Barnhart M, James D, Shelton JD. ARVs: The next generation. Going boldly together to new frontiers of HIV treatment. Glob Health Sci Pract. 27 January, 2015. http://www.ghspjournal.org/content/3/1/1
3. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: what’s new. Policy brief. Geneva: World Health Organization. November 2015. Available: http://www.who.int/hiv/pub/arv/policy-brief-arv-2015/en/
4. Clayden P. Fit for purpose: antiretroviral treatment optimisation. HIV treatment bulletin. July 2015. http://i-base.info/htb/28509
5. 't Hoen EF, Hogerzeil HV, Quick JD, Sillo HB. A quiet revolution in global public health: The World Health Organization's Prequalification of Medicines Programme. J Public Health Policy. 2014 May;35(2):137-61.
6. Pérez Casas C. Accelerating access for optimized regimens. Presentation at WHO ICASA Non-Abstract Driven Session HIV Treatment: What's new in the pipeline. 30 November 2015.
7. Expert review panel procedure: additional support to procurement agencies under exceptional circumstances. Geneva: World Health Organization. September 2013. Available: http://apps.who.int/prequal/info_general/documents/ERP/Expanded_ERP_process_Sep2013.pdf
NOTES
1. This is why UNITAID is currently the main funder of the WHO prequalification programme for medicines and diagnostics for HIV, TB and malaria.
2. In countries that apply data exclusivity, generic manufacturers would be required to conduct their own clinical trials in order to obtain marketing approval, or to wait until a specified exclusivity period (which varies among countries but often lasts five to ten years) has passed before a generic product can be approved.
3. UNITAID has been instrumental in incentivizing the development of paediatric formulations for HIV and TB.
4. UNITAID funds an important part of the WHO prequalification programme for medicines and diagnostics for HIV, TB and malaria.
5. UNITAID is the sole funder of the Medicines Patent Pool. See also UNITAID’s first contribution to the High Level Panel. More information can be found at http://www.medicinespatentpool.org/
6. UNITAID does not fund basic research or early stage development, but does fund certain projects that aim to develop new formulations of existing medicines, or the late-stage development of new diagnostics.